Electrochemical performances of LiFePO4/C composites prepared by molten salt method
CHEN Zhao-yong(陈召勇), ZHU Wei(朱 伟), ZHU Hua-li(朱华丽),
ZHANG Jian-li(张建利), LI Qi-feng(李奇峰)
College of Physics and Electronic Science, Changsha University of Science and Technology,
Changsha 410114, China
Received 29 October 2009; accepted 1 March 2010
Abstract:
LiFePO4/C composites were synthesized by a molten salt (MS) method using the mixture of LiCl, LiOH and NaCl. The prepared LiFePO4/C composites are characterized by X-ray diffractometry (XRD), field emission scanning electron microscopy (FESEM) and charge-discharge test. XRD patterns indicate that LiFePO4 prepared in the temperature range of 550-700 ℃ crystallizes well in an olivine-type structure.
Key words:
lithium iron phosphate; molten salt method; cathode material; Li-ion batteries;
1 Introduction
Olivine-type LiFePO4 has been one of the most promising candidates as cathode intercalation material for lithium-based secondary batteries since it was reported by PADHI et al[1]. Compared with conventional cathode materials, such as LiCoO2 and LiNiO2, it shows many advantages, such as nontoxicity, low cost, excellent structural stability and high theoretical capacity (170 mA·h/g). So, it is suitable to be used as the cathode material for the large-size lithium battery in the electric and hybrid electric vehicles (EV & HEV). However, its poor rate capability at high rates during the charge-discharge process due to the low intrinsic electronic conductivity and poor lithium ion diffusion becomes an obstacle to be applied extensively[2-3]. To overcome this limitation, a lot of efforts have been paid. Carbon coating is one of the efficient methods which can improve the surface electronic conductivity of active particles[4]. Super valence metal ion doping on Li-site or Fe-site is another way to enhance the intrinsic electronic conductivity or the lithium ion diffusion[5]. According to the model of ANDERSSON and THOMAS[6], producing small particles is also an available technique that can amend the electrochemical performance. Small particles can be synthesized by a variety of material processing methods, such as sol-gel method, hydrothermal method and liquid-state co-precipitation method[7-9]. Recently, the molten salt method (MS) has been widely employed to manufacture nano-materials, including cathode materials for lithium-based secondary batteries[10-12]. The small particles can be obtained at relatively low temperatures due to the high ion diffusion rates between reaction components in the molten media[13]. Olivine-type LiFePO4/C has prepared by MS in this work, and its electrochemical performances are studied.
2 Experimental
2.1 Preparation of LiFePO4/C
LiFePO4/C was synthesized by the molten salt method from stoichiometric amounts of LiCl, LiOH·H2O, FePO4·2H2O and NaCl in a molar ratio of 0.63?0.37?1?4. A certain amount of C6H12O6·H2O as carbon source was added. The precursors were mixed in a ball-milling machine at 500 r/min for 4 h with anhydrous ethanol as the dispersion medium. Then, the milled mixture was dried at 60 ℃ and calcined at a set temperature between 550 ℃ and 700 ℃ for 12 h. Finally, the products were washed completely with de-ionized water and anhydrous ethanol, filtered and dried in vacuum.
2.2 Characterization
Thermogravimetric (TG) and differential scanning calorimetry (DSC) analyses were carried out on a simultaneous thermal analysis apparatus (NETZSCH STA409PC, Germany) to determine the sintering temperature. The milled mixtures were heated from room temperature to 750 ℃ at a heating rate of 5 ℃/min under nitrogen flow.
The phase purity and structure were characterized by X-ray diffractometry (XRD, D/max-2000 Rigaku, Japan) with Cu Kα radiation (l=1.540 56 ?) operating at 40 kV and 40 mA. The scan range was 10?<2θ<90?, and a step of 0.01? was used.
The morphology was observed with field emission scanning electron microscopy (FESEM, Sirion, Holland). Transmission electron microscopy (TEM, Tecnai G 20 ST) observation was conducted in order to reveal the location of amorphous carbon, and the combined electron diffraction spectroscopy (EDS) investigation was performed for analyzing the spatial distribution of carbon element in the samples.
The content of carbon was investigated on the high-frequency infrared carbon-sulfur analyzer apparatus (CS-600, Leco Corporation). The corresponding carbon contents were 6.01%, 6.06%, 6.04% and 6.03% in the samples prepared at 550, 600, 650 and 700℃, respectively. The contents of Fe and P were determined by chemical analysis (YS/T53P.6-2006, Ammonium phosphate precipitation-acid-base titration), and the contents of other elements, such as Li, Na and K, were detected by inductively coupled plasma atomic emission spectrometry (ICP-AES, IRIS Advantage 1000).
2.3 Charge-discharge test
The positive electrode consisted of 80% as-prepared composites, 10% acetylene black and 10% polyvinylidene fluoride (PVDF) as a binder, and metal Al was used as collector. Celgard 2400 was used as separator which was soaked in 1.0 mol/L LiPF6/EC+DMC (EC?DMC=1?1 in volume ratio) electrolyte. Lithium metal foil was used as the counter electrode during electrochemical measurements. All cells were assembled in an argon-filled glove box. The charge-discharge tests were carried out by using a Land-BTL10 automatic battery test system. If not specified, the charge-discharge test was completed at 2.5-3.9 V and the density of current was 0.1C (17 mA/g).
Cyclic voltammetry was carried out on a CHI660B electrochemical working station (Chenhua, Shanghai, China) between 2.0 and 4.5 V at a scanning rate of 0.1 mV/s.
3 Results and discussion
3.1 TG-DSC analysis
Fig.1 shows the TG-DSC curves of the precursors used to prepare LiFePO4/C. Mass loss takes place from room temperature to 500 ℃. The mass loss in the range of 20-500 ℃ is 22.29%, which is attributed to the degradation of C6H12O6?H2O, FePO4?2H2O and LiOH·H2O and the reaction between FePO4 and LiOH. There are two obvious endothermic peaks and a clear exothermic peak on DSC curve. The values of the peak position are 125.5 ℃, 170 ℃ and 424.5 ℃. The two endothermic peak positions correspond to the mass loss on TG curve. The clear exothermic peak at 424.5 ℃ may be attributed to LiFePO4 transforming from amorphous form to crystalline[14-15]. Therefore, it is reasonable to set the sintering temperature between 500 and 750 ℃. Since the ion diffusion rates of the components in molten salts are much higher than those in the solid-state reaction, it probably takes less time to carry out the reaction. So, the heating time is also shortened to 12 h compared with the traditional solid state method, in which the precursor is usually sintered for more than 24 h[16].
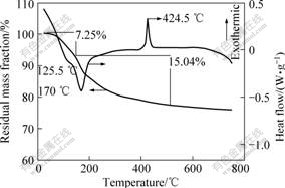
Fig.1 TG-DSC curves of reaction precursors
3.2 XRD analysis
Fig.2 shows the X-ray diffraction (XRD) patterns of the LiFePO4/C prepared at different temperatures. All samples are mainly identified as an olivine-type LiFePO4 with space group of Pnmb (JCPDS card No. 83-2092) and minor NaFePO4 (JCPDS card No. 29-1216) phase is also detected in the LiFePO4/C composites. Carbon phase is not found, and it maybe exists in amorphous form.
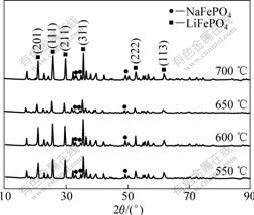
Fig.2 XRD patterns of LiFePO4/C composites
3.3 Electrochemical performance of LiFePO4/C composites
Fig.3 shows the charge and discharge curves of LiFePO4/C prepared at different temperatures for 12 h, all of which exhibit good electrochemical performances. The first discharge capacities of LiFePO4/C prepared at 550, 600, 650 and 700 ℃ are 114.7, 136.3, 150.8 and 108.6 mA?h/g, respectively. The LiFePO4/C composites prepared at 600 ℃ manifest the highest average discharge voltage and good shape among all the prepared samples. The average discharge voltages of LiFePO4/C prepared at 550, 600, 650 and 700 ℃, are around 3.28, 3.36, 3.34 and 3.29 V, respectively. It may be attributed to the fact that the LiFePO4/C powder prepared at 600 ℃ has better crystallinity than the samples prepared at lower temperatures, and smaller particle size than that prepared at 700 ℃[17]. So, an appropriate temperature must be carefully selected to prevent the undesirable particle growth and the presence of a noncrystalline phase.
Fig.4 shows the cycle performance of the LiFePO4/C composites prepared at 600 ℃ and 650 ℃ at the rates of 0.1C, 0.2C and 0.5C at room temperature.
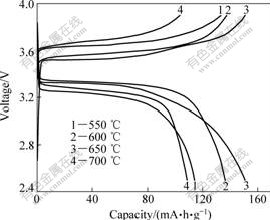
Fig.3 Charge-discharge curves of LiFePO4/C composites
The LiFePO4/C composites prepared at 600 ℃ and 650 ℃ show comparable specific discharge capacities of 136.3 and 150.8 mA?h/g at 0.1C, 144.6 and 135.4 mA?h/g at 0.2C, and 122.3 and 111.8 mA?h/g at 0.5C, respectively. The LiFePO4/C composites prepared at 600 ℃ exhibit good electrochemical performances at 0.5C, while obvious capacity loss is observed for other samples. The reason could be the higher content of Na+ in the sample prepared at 650 ℃. According to XRD and ICP-AES analyses, NaFePO4 phase are detected and the contents of Na+ are 0.18% and 0.56% in the two samples prepared in 600 ℃ and 650 ℃, respectively. The presence of Na+ in the LiFePO4 crystal lattice could be harmful to the transmission of Li+. The mechanism is still to be explored in the following work.
Fig.5 shows the cyclic voltammetry curves of the LiFePO4/C composites prepared at 600 ℃. The oxidation and reduction peaks appear at around 3.694 and 3.106 V, respectively, with the potential interval of 0.588 V. The peak of profiles are rather symmetric and spiculate, indicating a good cycle reversibility.
3.4 Morphology observation
Fig.6 shows the FESEM images of the LiFePO4/C
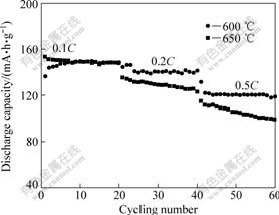
Fig.4 Cycle performances of LiFePO4/C composites
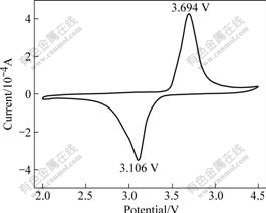
Fig.5 Cyclic voltammograms of LiFePO4/C composites (Scan rate 0.1 mV/s)
composites prepared at different temperatures in LiOH-LiCl-NaCl molten system. It is very obvious that the LiFePO4 particles gradually grow up from 0.2 to 1.5 μm when the calcination temperature increases from 550 to 700 ℃. Those amorphous carbons, which are flaky in most cases, continuously distribute among homogeneous LiFePO4 particles. In these cases of 550, 650 and 700 ℃, smoothly-edged polyhedral LiFePO4 particles are observed, while they do not exist in the sample prepared in 600 ℃. Even so, in all these cases most particles are in spherical shape. With respect to the charge-discharge properties (Fig.4), it seems that the spherical shape of LiFePO4 contributes to the better cycling behavior. The particles of LiFePO4/C prepared by conventional solid-state reaction present the irregular geometry shape and aggregate seriously[18], the size of which is larger than that of samples prepared by MS method. It is also indicated that the presence of LiOH-LiCl-NaCl contributes to hindering the aggregation of LiFePO4 particles. In the temperature range of 550-700 ℃, the growth behavior of LiFePO4 particles in the LiOH-LiCl-NaCl may be similar with that in aqueous solution. Spherical shape morphology may result from the higher ion diffusion rate, rapid heat exchange and strong tensile force from the molten salt.
Fig.7 shows the TEM image and EDS analysis results of the LiFePO4/C composites prepared at 600 ℃ in LiOH-LiCl-NaCl molten system. In Fig.7, the EDS analysis corresponds to the chemical composition of locations 1 and 2. It can be obviously observed that some amorphous carbons coat on the surface of the LiFePO4 particles, and other dissociative carbons interconnect to form network, which contributes to the enhanced electric conductivity.
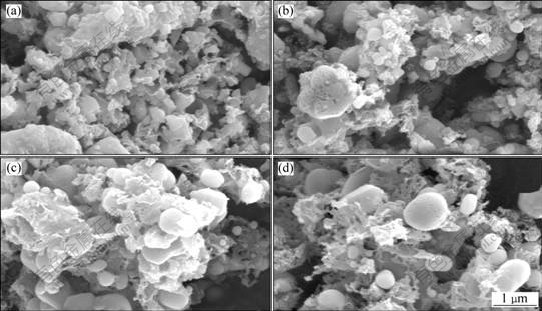
Fig.6 FESEM images of LiFePO4/C composites prepared at different temperatures in LiOH-LiCl-NaCl molten systems: (a) 550 ℃; (b) 600 ℃; (c) 650 ℃; (d) 700 ℃
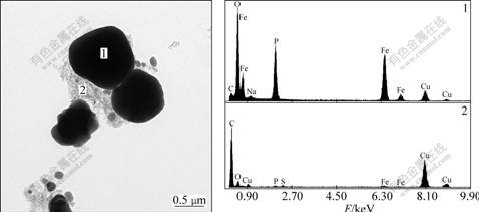
Fig.7 TEM image and EDS spectra of LiFePO4/C composites prepared at 600 ℃
4 Conclusions
1) Well-crystallized lithium iron phosphate has been successfully synthesized by molten salt method. The temperature of LiFePO4 transforming from amorphous form to crystalline in LiOH-LiCl-NaCl molten system is 424.5 ℃. Compared with the solid-state reaction, LiFePO4 synthesized by molten salt method requires lower temperature and shorter synthesized time.
2) Sphere-like and homogeneous particles of 0.2 μm can be observed through FESEM and TEM images.
3) LiFePO4 synthesized at 600 ℃ for 12 h exhibits good electrochemical performance. At the rate of 0.2C (34 mA/g) and 0.5C, the discharge capacities are 144.6 and 122.3 mA?h/g, respectively, together with good cycle performances.
References
[1] Padhi A K, Nanjundaswamy K S, Goodenough J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries [J]. Journal of the Electrochemical Society, 1997, 144: 1188-1193.
[2] Kwon S J, Kim C W, Jeong W T, Lee K S. Synthesis and electrochemical properties of olivine LiFePO4 as a cathode material prepared by mechanical alloying [J]. Journal of Power Sources, 2004, 137: 93-99.
[3] Gao Fei, Tang Zhi-yuan. Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries [J]. Electrochimica Acta, 2008, 53: 5071-5075.
[4] Cho Y D, Feya G T K, Kao H M. The effect of carbon coating thickness on the capacity of LiFePO4/C composite cathodes [J]. Journal of Power Sources, 2009, 189: 256-262.
[5] Chung S Y, Bloking J T, Chiang Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nature Materials, 2002, 1: 123-128.
[6] Andersson A S, Thomas J O. The source of first-cycle capacity loss in LiFePO4 [J]. Journal of Power Sources, 2001, 98: 498-502.
[7] Chen Zhao-yong, Zhu Hua-li, Ji Shan, Fakir R, Linkov V. Influence of carbon sources on electrochemical performances of LiFePO4/C composites [J]. Solid State Ionics, 2008, 179: 1810-1815.
[8] Jin E M, Jin B, Jun D K, Park K H, Gua H B, Kim K W. A study on the electrochemical characteristics of LiFePO4 cathode for lithium polymer batteries by hydrothermal method [J]. Journal of Power Sources, 2008, 178: 801-806.
[9] Yang M R, Ke W H, Wu S H. Preparation of LiFePO4 powders by co-precipitation [J]. Journal of Power Sources, 2005, 146: 539-543.
[10] Lala S M, Montoro L A, Rosolen J M. LiCoO2 sub-microns particles obtained from micro-precipitation in molten stearic acid [J]. Journal of Power Sources, 2003, 124: 118-132.
[11] Ni Jiang-feng, Zhou Heng-hui, Chen Ji-tao, Zhang Xin-xang. Molten salt synthesis and electrochemical properties of spherical LiFePO4 particles [J]. Materials Letters, 2007, 61: 1260-1264.
[12] Ha H W, Jeong K H, Kim K. Effect of titanium substitution in layered LiNiO2 cathode material prepared by molten-salt synthesis [J]. Journal of Power Sources, 2006, 161: 606-611.
[13] Han C H, Hong Y S, Park C M, Kim K. Synthesis and electrochemical properties of lithium cobalt oxides prepared by molten-salt synthesis using the eutectic mixture of LiCl-Li2CO3 [J]. Journal of Power Sources, 2001, 92: 95-101.
[14] Wu S H, Hsiao K M, Liu W R. The preparation and characterization of olivine LiFePO4 by a solution method [J]. Journal of Power Sources, 2005, 146: 550-554.
[15] Zhang Ming, Jiao Li-fang, Yuan Hua-tang, Wang Yong-mei, Guo Jian, Zhao Ming, Wang Wei, Zhou Xing-di. The preparation and characterization of olivine LiFePO4/C doped with MoO3 by a solution method [J]. Solid State Ionics, 2006, 177: 3309-3314.
[16] TIAN Yan-wen, KANG Xiao-xue, LIU Li-ying, XU Cha-qing, QU Tao. Research on cathode material of Li-ion battery by yttrium doping [J]. Journal of Rare Earths, 2008, 26(2): 279-283.
[17] Xu Yan-bin, Lu Ying-jun, Yan Lan, Yang Zheng-yin, Yang Ru-dong. Synthesis and effect of forming Fe2P phase on the physics and electrochemical properties of LiFePO4/C materials [J]. Journal of Power Sources, 2006, 160: 570-576.
[18] Kima D K, Park H M, Jung S J, Jeong Y U, Lee J H, Kima J J. Effect of synthesis conditions on the properties of LiFePO4 for secondary lithium batteries [J]. Journal of Power Sources, 2006, 159: 237-240.
(Edited by YANG Bing)
Foundation item: Project(06B002) supported by the Scientific Research Fund of Hunan Provincial Education Department of China; Project(09JJ3092) supported by the Natural Science Foundation of Hunan Province, China; Project(2008FJ3008) supported by the Planned Science and Technology Project of Hunan Province, China
Corresponding author: CHEN Zhao-yong; Tel:+86-731-85258234; Fax: +86-731-85258217; E-mail: chenzhaoyongcioc@126.com
DOI: 10.1016/S1003-6326(09)60218-1



