Electropolishing parameters of NiTi alloy
MIAO Wei-dong(缪卫东)1, MI Xu-jun(米绪军)2, WANG Xin-lu(王新录)1, LI Hua-chu(栗华矗)1Li Rui (李锐)
1. General Research Institute for Nonferrous Metals, Beijing 100088, China;
2. State Key Laboratory for Fabrication and Processing of Nonferrous Metals, Beijing 100088, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Electropolishing has been used in NiTi alloy in several fields for its special characteristics, but its essential details and electropolishing mechanism have not been reported yet as a demand from business competition, which, to a great degree, restricts the application and extension of the electropolishing technology. The effect of processing parameters on nitinol electropolishing was explored. Besides the electrolyte, other factors that influence the electropolishing are temperature, current density, time, spacing between anode and cathode, electrolyte stirring, etc. Studies on the effect of the temperature on the electropolishing process show that the higher the temperature is, the bigger the electropolishing rate is, following the near Gauss law. The relationship between the temperature and the surface roughness follows a near parabolic law, and the relationship between the temperature and the surface reflectivity follows a near sigmoidal law. The relationship between the electropolishing voltage and the current density follows a near cubic law, while that between the electropolishing rate and current density follows a near linear law. The relationship between the electropolishing rate and the time follows a near sigmoidal law. The practical spacing between anode and cathode is confirmed by the Hall bath experiment.
Key words:
NiTi alloy; nitinol; electropolishing; processing parameters;
1 Introduction
The shape memory alloy NiTi has been a candidate biomaterial for several years due to its unique thermal shape memory effect, superelasticity, resistance to fatigue and corrosion and high damping properties. It is in common use, for example, self-expanding stents, graft support systems, filters, baskets and various other devices for minimally-invasive interventional and endoscopic procedures[1-3]. In recent years, the study of surface preparation has attracted considerable attention due to its potentially important effect on properties of NiTi alloy. Among those surface treatment methods, electropolishing has attracted a great attention for its distinctive advantages. Electropolishing removes slag, burrs, scratches, heat-affected zones and creates a new, smoother surface. The polishing rate can be carefully adjusted within tight tolerances to meet different requirements. Electropolished NiTi alloy is covered by a thin (about 3nm) oxide film which is responsible for the outstanding corrosion resistance and biocompatibility. The polishing process results in a protective film consisting of pure titanium oxide. The entire nickel free surface has a clear advantage compared to mechanically polished surfaces[4-7].
Thanks to those advantages of electropolishing, it has been applied in NiTi alloy in many fields, especially in medical field. But few mechanism and technics of NiTi alloy have been reported in detail due to the business competition[8, 9].
In this paper, the effect of processing parameters on electropolishing of NiTi alloy was analyzed, and it was expected that our work would be beneficial to optimizing the electropolishing parameters of NiTi alloy.
2 Experimental
A nearly equiatomic NiTi alloy with an analysed composition of 55.6%Ni (mass fraction, the same below) was prepared from the raw materials of titanium (purity 99.9%) and nickel (purity 99.97%) with vacuum inductive melting technique being used. The ingot was then forged and rolled to sheets of 0.5 mm in thickness. The Af temperature of the sheets measured by DSC was about 10 ℃. All samples were mechanically polished with successive emery papers down to No.400 producing a similar finish with an original surface roughness (Ra: 216 nm) and original surface reflectivity (Re: 47.68%).
In a special solution, samples were electropolished with DP-1 electropolishing instrument at different temperatures by ZL-L-1800 semiconductor refrigeration system. The surface roughness of samples was measured by the MHT-Ⅲ non-contact three-dimensional surface topography measuring instrument and the surface reflectivity was measured by the CS5 computer color matching system.
3 Results and discussion
The optimum temperature of NiTi alloy was investigated in detail by reviewing the effects of the electropolishing temperature on the electropolishing rate (v), surface roughness (Ra) and surface reflectivity (Re). The other parameters were: electropolishing time 45 s, spacing between anode and cathode 1.8 cm, current density 0.7 A/cm2.
v=m/(t×S)
where m is the mass of metal removal, t is the electropolishing time, S is the metal surface area.
It can be seen from Fig.1 that the higher the electrolyte temperature is, the bigger the electro- polishing rate is, following a near Gauss law, which is in accordance with the results of studying the relationship between the electropolishing temperature and electropolishing with surface roughness method by MATLOSZ [10].

Fig. 1 Effect of temperature on electropolishing rate
It can be seen from Fig.2 that the relationship between the temperature and surface roughness follows a near parabolic law, the relationship between the temperature and surface reflectivity follows a near sigmoidal law. The optimum temperature of our electrolyte should be controlled between 0-10 ℃.
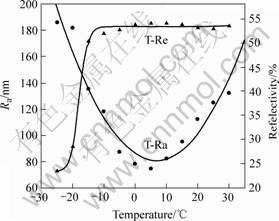
Fig.2 Effect of electropolishing temperature on surface roughness and surface reflectivity
The experiments show that the relationship between the electropolishing voltage and current density follows a near cubic law while that between the electropolishing rate and current density follows a near linear law (Fig.3). The better surface can be gotten when the current density is about 0.7 A/cm2.

Fig.3 Electropolishing current density vs voltage and electro- polishing rate
It is found that the electropolishing characteristic curve (voltage vs current density curve) of NiTi alloy doesn’t show electric current plateau, which is very different from the typical one of the common metal[11]. When the specimen is electropolished at the lower potential, the speed of electropolishing is controlled by the diffusion rate of reaction products passing the viscous layer, while at the higher potential, the electropolishing mechanism is mainly pitting erosion.
The effect of electropolishing time on electro- polishing rate was studied. From Fig.4 it can be found that the relationship between the electropolishing rate and time follows a near sigmoidal law. Prolonging the processing time unprincipledly is unnecessary and harmful.

Fig.4 Effect of electropolishing time on electropolishing rate
The spacing between anode and cathode is confirmed by the Hall bath experiment, as shown in Fig.5. According to the surface characteristics of different regions of the result in Hall bath experiment on NiTi samples, the practical spacing (1.6-3.0 cm) between anode and cathode for better surface can be gotten.
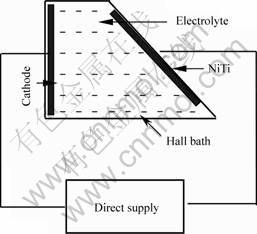
Fig.5 Schematic diagram of Hall bath experiment
With the process of electropolishing, anodic solution products increase gradually and assemble on the surface of the anode which will influence the normal electropolishing process[12]. So during the electro- polishing, the mechanical agitation was adopted to enforce the electrolyte convecting, remove the anodic products assembling on the anode, reduce the polarization caused by the concentration difference, so the electropolishing temperature can be more stable to guarantee the optimal electropolishing condition. Moreover, agitation can accelerate the dissolution of the anodic membranous, impel the bladders attached to the sample surface to transgress out, then increase the electric current and enhance the efficiency.
4 Conclusions
Besides the electrolyte, other factors that influence the electropolishing are temperature, current density, spacing between anode and cathode, electrolyte stirring, etc. Studies on the effect of the temperature on the electropolishing process show that the higher the temperature is, the bigger the electropolishing rate is, following a near Gauss law. The relationship between the temperature and surface roughness follows a near parabolic law, and the relationship between the temperature and surface reflectivity follows a near sigmoidal law.
The relationship between the electropolishing voltage and current density follows a near cubic law, while that between the electropolishing rate and current density follows a near linear law. The relationship between the electropolishing rate and time follows a near sigmoidal law. The practical spacing between anode and cathode is confirmed by the Hall bath experiment and the agitation is necessary during the process for a better surface.
References
[1] SHABALOVSKAYA S. Shape Memory Materials and Its Applications. Materials Science Forum 394-395 [M]. Switzerland: Trans Tech Publications Ltd, 2002. 131-144.
[2] RYHANEN J, NIEMI E, SERLO W, et al. Biocompatibility of nickel-titanium shape memory metal and its corrosion behavior in human cell cultures[J]. J Biomed Mat Res, 1997, 35: 451-457.
[3] SHABALOVSKAYA S A. Physicochemical and biological aspects of Nitinol as a biomaterial [J]. International Metals Reviews, 2001, 46(5): 233-250.
[4] WEVER D J. Electrochemical and surface characterization of a nickel-titanium alloy [J]. Biomat, 1998. 19: 761-769.
[5] HUNT N P, GOLDEN CG, SHERIFF M. An investigation into the effects of polishing on surface hardness and corrosion of orthodontic archwires [J]. Angle Orthod, 1999, 69: 433-440.
[6] FIRSTOV G S, KUMAR H. Surface oxidation of NiTi shape memory alloy [J]. Biomaterials, 2002, 23: 4863-4871.
[7] GENG Fang, SHI Ping, CHENG F T. TiO2 film preparation and hemocompatibility of NiTi shape memory alloy [J].The Chinese Journal of Nonferrous Metals, 2004, 14(9): 1575-1579.
[8] ASLANIDIS D. Electropolishing for Medical Devices:Relatively New Fascinatingly Diverse [M]. Switzerland: Trans Tech Publications Ltd, 2002.169-172.
[9] Admedes Schuessler GmbH. Electropolishing of Nitinol [EB/OL]. http://www.nitinol.de/processes/electropolishing.htm. Jan. 11, 2006.
[10] MATLOSZ M. Effects of surface finish on the corrosion of Niti alloy for biomedical applications [J]. Journal of the Electrochemical Society, 1989, 136(4): 919-929.
[11] MIAO Wei-dong, MI Xu-jun, ZHU Ming. Study on Electropolishing Mechanism of NiTi Alloy [A]. LUTJERING G. Ti-2003 Science and Technology [C]. Germany: WILEY-VCH Verlag GmbH & Co. KGaA, 2004. 861-864
[12] WU Hui-huang. Electrochemistry [M]. Beijing, Chemistry Industry Press, 2004. 2-5.
Corresponding author: MIAO Wei-dong; Tel: +86-10-80103388-8307; Fax: +86-10-80105303; E-mail: richard_miao2008@yahoo.com.cn



