- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discussion▲
- 4 Conclusions▲
- References
- Figure
- Fig.1 Growth routes of aluminium hydroxide particles
- Fig.2 SEM images of precursors
- Fig.3 Particle size(a) and surface area(b) patterns of samples A , B and C
- Fig.4 SEM images of precursors
- Fig.5 Particle size(a) and surface area(b) patterns of samples
- Fig.6 TG-DTA curves of precursor
- Fig.7 XRD pattern of calcined product
- Fig.8 SEM image of calcined product
J. Cent. South Univ. Technol. (2007)06-0773-06
DOI: 10.1007/s11771-007-0147-4
![]()
Effect of anions on preparation of ultrafine α-Al2O3 powder
XIAO Jin(肖 劲)1, QIN Qi(秦 琪)1, WAN Ye(万 烨)2, ZHOU Feng(周 峰)1,
CHEN Yan-bin(陈燕彬)1, LI Jie(李 劼)1, LIU Ye-xiang(刘业翔)1
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. China Enfi Nonferrous Engineering Co. Ltd, Beijing 100038, China)
Abstract:
Ultrafine alumina power was obtained by calcining the precursor at 1 200 ℃ for 2 h, which was prepared by homogeneous precipitation method using aluminium salts and urea as raw materials. The effects of anions on the morphology, particle size, surface area and configuration of the precursors were studied. The results show that the reactions of urea with aluminium nitrate and aluminium chloride result in agglomerates gels with bad filtering performance, the morphology is fibrillar. Aluminium sulphate-urea reactions result in the direct formation of amorphous powders with good filtering performance, of which morphology are regular spherical particles with larger granularity and smaller surface area. The reaction of mutual compound of aluminium sulphate and aluminium nitrate with molar ratio of 40:60 with urea can produce precursor with good filtering performance, spherical morphology, and uniform granularity distribution in the particle size range of 2-3 μm.
Key words:
α-Al2O3; ultrafine powder; homogeneous precipitation; anion; mutual compound;
1 Introduction
Ultrafine alumina, with excellent properties such as high heat-resistance, high corrosion-resistance, good electrical insulation and catalytic abilities, is a very important branch of ultrafine powder materials and widely applied in fields of ceramics, metallurgy, electron and catalysts. Particularly, α-Al2O3 powder also has advantaged characterisitics, such as high surface area and strong absorbency, which is a very important structural and functional material widely applied in many fields[1-3]. In order to attain the fine molding and calcining characteristics, α-Al2O3 powder is acquired to have the characteristics of high purity, ultrafine particle size, regular morphology and narrow particle size distribution.
Various methods have been proposed to prepare ultrafine and near-monodisperse alumina particles[4-7]. These methods may be classified into three categories, that is solid, liquid and vapor methods. Solid-phase methods include thermal dissociation and mechanical milling; liquid-phase methods are based on homogeneous precipitation, hydrolysis, and sol-gel techniques; vapor condensation, vapor-vapor, vapor- liquid and vapor-solid reactions are typical examples of vapor-phase methods. Among these methods, it is generally agreed that liquid-phase methods are suitable to precisely control chemical compositions. Furthermore, the homogeneous precipitation method has an advantage in the preparation of ultrafine alumina particles with relatively narrow size distribution.
Though there are some reports on the preparation of the ultrafine α-Al2O3 powder by homogeneous precipitation[8-11], the effect of anions on the synthesis of the ultrafine α-Al2O3 powder has not been reported and there is not an accordant standpoint. In this study, the effect of anions on the preparation of the ultrafine α-Al2O3 powder was investigated[12-15].
2 Experimental
2.1 Experimental process
Aqueous solutions with desired concentrations of aluminium nitrate-urea, aluminium chloride and aluminium sulphate-urea were prepared and thermostatted at 100 ℃ in a round bottom flask. The concentration of aluminium was 0.05 mol/L at first, while the urea was 0.25 mol/L. During reaction the solution was stirred by a mechanical stirrer. After reacting for 2 h, the precursor was filtered, washed repeatedly with distilled water and dried in oven at 80 ℃ for 12 h, and then the dried precursor was calcined at 1 200 ℃ for 2 h to obtain α-Al2O3 powder.
2.2 Characterization of sample
The calcined products were identified using a Rigaku X-ray powder diffractometer (XRD) with Cu Kαradiation; the differential thermal analysis (DTA) and thermogravimetric analysis (TGA) curves of the precursors were recorded by TG-DTG-DTA instrument of model SDT Q600; the morphology of particles and the dispersion of the powders were characterized by JSM-5600LV scanning electron microscope (SEM), the powders for SEM observation were scattered in acetone ultrasonically and then the suspended powders were mounted on a copper microgrit; the surface area of the precursor was characterized by ST-03 surface aperture cryoscope; the particle size of the precursor was characterized by BT-2001 laser particle size analyzer.
3 Results and discussion
3.1 Reaction mechanism
The routine neutralisation and deposition reactions happen locally in the reaction system. The qualification is quite asymmetry. The neutralisation and deposition take place simultaneously in the reaction system when the urea is used as precipitator, which keeps the equality character of the primary deposited particles. The reaction mechanism of the formation of alumina particles through homogeneous precipitation includes two distinct steps: decomposition of urea and precipitation reaction.
Decomposition of urea:
CO(NH2)2+3H2O=2NH3·H2O+CO2↑ (1)
NH3·H2O=NH4+ + OH- (2)
Precipitation reaction:
Al3++3OH-=Al (OH) 3↓ (3)
3.2 Effect of Cl-, SO42- and NO3- on preparation of ultrafine alumina
Table 1 shows the appearance of the three precursors and the terminating pH value of reaction by different aluminium salts. During the reaction, the concentration of Al3+ is 0.2 mol/L, molar ratio of Al3+ to urea is 1:5, temperature is 100 ℃, and reaction time is 2 h. The most important point observed is that aluminium nitrate-urea and aluminium chloride-urea reactions initially result in a sudden formation of gelatinous precipitate at a pH value of 6.5, while sulphate-urea reaction produces compact granular precipitate (powders) at a pH value of about 4.2. In the further research, it is found that the terminating pH value of reaction is not influenced by the reactant concentrations, but influenced by anions. This is similar to the conclusion of ADA et al[16].
In the presence of NO3- or Cl-, because of the poor coordinate bond forming ability, NO3- and Cl- do not interfere in the reaction of hydroxide with the aluminium containing polymeric cation growing into the larger charged ploymeric species via olation and oxolation within Al-O-Al bridges, with increasing pH value of about 6.5 (RouteⅠin Fig.1). While in the presence of SO42-, due to the better coordinating ability of sulphate bonds to the growing polymeric cation, terminating further polymerization results in neutral species of composition Al4(OH)10SO4. The precipitation occurs slowly at the low pH value and particles grow slowly, resulting in spherical particles (Route Ⅱin Fig.1).
Table 1 Appearance of three precursors and reaction terminating pH value by different aluminium salts


Fig.1 Growth routes of aluminium hydroxide particles
The SEM images of precursors A, B and C, which are synthesized with different aluminium salts, are shown in Fig.2. From Fig.2, it can be concluded: 1) the morphologies of precursor from aluminium sulphate-urea reaction are individual and amorphous, and the morphology of precursor from aluminium nitrate-urea and aluminium chloride-urea reaction is elongated fibrillar bundle; 2)the particle size of the precursor by aluminium sulphate-urea reactions (Fig.2(a)) is larger than that prepared by aluminium nitrate-urea and aluminium chloride-urea reactions (Figs.2(b) and (c)); 3) the particles prepared by aluminium sulphate-urea reactions are quite loose, which have better decentralization characteristics, while the precursors prepared by other aluminium salts(aluminium nitrate and aluminium chloride) are coagulated, leading to bad decentralization characteristic.
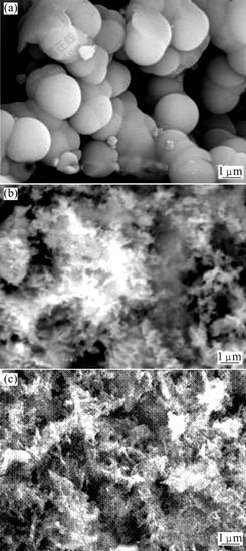
Fig.2 SEM images of precursors
(a) Sample A; (b) Sample B; (c) Sample C
The plots of particle size and surface area of precursors synthesized with three different kinds of aluminium salts are shown in Fig.3. It is clear that the precursor synthesized by aluminium sulphate has large particle size and small surface area with narrower particle size distribution. The precursors prepared by other aluminium salts (aluminium nitrate and aluminium chloride) have large surface area and small particle size with wider particle size distribution. Specially, compared with aluminium chloride, the precursor prepared by aluminium nitrate has narrower particle size distribution and larger surface area, which is preponderant.
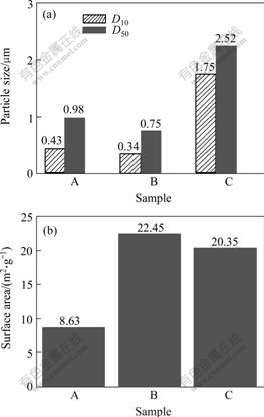
Fig.3 Particle size(a) and surface area(b) patterns of samples A , B and C
In order to gain alumina powder with spherical morphology, excellent decentralization characteristics, small particle size and uniform particle size distribution, the preparation process of precursor is vital. From the above, it can be concluded that when aluminium sulphate is used as the material, the precursor has narrow particle size distribution and excellent decentralization characteristic, and is filterable. However, the particle size is quite large and the surface area is small; when aluminium nitrate is used, the production has small particle size and large surface area, but with wide particle size distribution, and is difficult to filter because it is colloid. So in this work, the mutual compound of alumimium sulphate and alumimium nitrate was used as the material to carry out homogeneous reaction with urea. The resulting product, ultrafine alumina powder, is of the characteristic of small particle size, narrow particle size distribution, good filterability and excellent decentralization.
3.3 Effect of mutual compound of SO42- and NO3- on preparation of ultrafine alumina
3.3.1 Effect of mutual compound of SO42- and NO3- on configuration and morphology of precursor
The mutual compound of SO42- and NO3- is prepared under conditions that the molar ratios of SO42- to NO3- are 10?90, 20?80, 30?70 and 40?60, respectively, by which the precursors were the samples A′, B′, C′, D′. During the reaction the concentration of Al3+ is 0.2 mol/L; molar ratio of Al3+ to urea is 1?5; temperature is 100 ℃; time is 2 h. Table 2 shows the appearance of the four precursors and the terminating pH value of reaction.
Table 2 Appearance of four precursors and reaction terminating pH value at different molar ratios of SO42- to NO3-
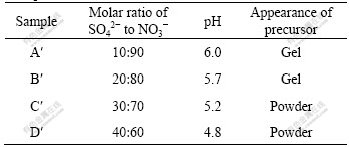
When the molar ratio of SO42- to NO3- is 10:90, the gelation occurs at a pH value of 6.0; when the molar ratio of SO42- to NO3- is 20:80, the gelation occurs at a pH value of 5.7; when the molar ratio of SO42- to NO3- is 30:70, the precursor is compact granular precipitate (powders) obtained at a pH value of 5.2; when the molar ratio of SO42- to NO3- is 40:60, the precursor is compact granular precipitate gained at a pH value of 4.8. It is concluded that with increasing the molar ratio of SO42- to NO3-, the configuration of the precursor becomes filterable, and the colloid becomes the precipitation. Therefore, SO42- is helpful for decreasing the pH value of precursor forming.
The SEM images of precursors A′, B′, C′ and D′ are shown in Fig.4. It is clear that the morphologies of precursors change from the elongated fibrillar bundles (Fig.4(a)) to amorphous powders with increasing molar ratio of SO42- to NO3-, which is better than the precursor prepared by aluminium nitrate with the loosen characteristics (Fig.2(b)); when the molar ratio of SO42- to NO3- reaches 20:80, the morphology of the precursor becomes from fibre to sphericity (Fig.4(b)); when the molar ratio of SO42- to NO3- reaches 30:70, the spherical morphology of precursor is inerratic (Fig.4(c)), but the particle size distribution is asymmetry; when the molar ratio of SO42- to NO3- reaches 40?60, the product consists of regular granular precipitates (Fig.4(d)), with uniform particle size distribution. So SO42- is helpful for the morphology of precursor to change into sphericity. And the larger the molar ratio of SO42- to NO3- is, the more inerratic the spherical morphology becomes.
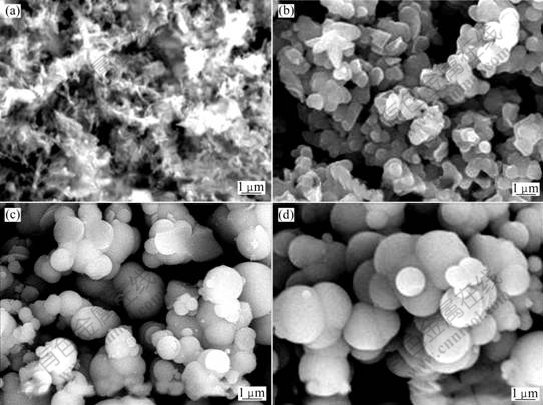
Fig.4 SEM images of precursors
(a) Sample A′; (b) Sample B′; (c) Sample C′; (d) Sample D′
3.3.2 Effect of mutual compound of SO42- and NO3- on surface area and particle size of precursor
The particle size and surface area of the four precursors A′, B′, C′ and D′ are shown in Fig.5. It can be
concluded that with the increase of SO42-, the particle size increases and the surface area decreases. But compared with the precursor prepared by aluminium sulphate (Fig.2(a)), the particle size decreases and the surface area increases, which indicates that the mutual compound of anions can decrease the particle size and increase the surface area effectively.
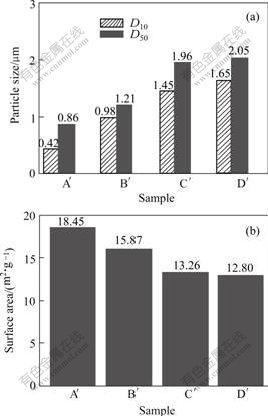
Fig.5 Particle size(a) and surface area(b) patterns of samples
3.4 Affirmation of optimized sample
Based on the experimentation, it can be concluded that the optimized testing conditions are as follows: mutual compound of aluminium sulphate and aluminium nitrate with molar ratio of 40:60, is used as raw materials; the concentration of Al3+ is 0.05 mol/L, the concentration of the urea is 0.25 mol/L; the reaction is carried out at 100 ℃ for 2 h. During the reaction, the solution is stirred mechanically. The resulting product is produced after the precursor is washed with distilled water repeatedly and dried at certain temperature, and then calcined at 1 200 ℃.
The TG-DTA measurement was performed to
confirm the species of the precursors. In Fig.6, it can be seen that when the temperature is lower than 600 ℃, the mass loss of the precursor is a continuous process, the TG curve declines continuously; when the temperature is higher than 600 ℃, the TG curve is horizontal, which indicates that the mass loss of the decomposition is ended. At this moment, the mass loss of the precursor is about 34%, which is anastomosed with the theoretical mass loss ratio (34%) from Al(OH)3 to Al2O3. So it can be concluded that the precursor is pure Al(OH)3.
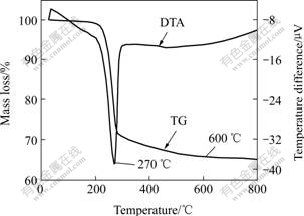
Fig.6 TG-DTA curves of precursor
The XRD pattern of the calcinated product is shown in Fig.7. It is clearly observed that diffraction peaks of the calcinated product agree with the PDF11-0661. It is deduced that the calcinated product is pure α-Al2O3, the peak intensities in Fig.7 show the characteristics of perfect crystal and high purity. Fig.8 shows the SEM image of the resulting product (ultrafine alumina powder), indicating that the product is of spherical morphology and uniform particle size distribution with average particle size of 2-3 μ m.
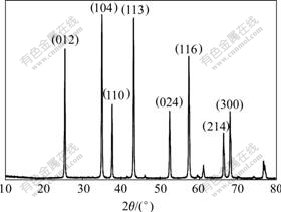
Fig.7 XRD pattern of calcined product

Fig.8 SEM image of calcined product
4 Conclusions
1) The ultrafine alumina powder is prepared by homogeneous precipitation method using three kinds of aluminium salts and urea as raw materials. The anions are very important to the configuration, morphology, particle size and surface area of the precursors and are vital to the terminating pH value of the reaction. Both aluminium nitrate-urea and aluminium chloride-urea reactions result in an amorphous gel, which produces the elongated fibrillar morphology with bad dispersing performance. Aluminium sulphate-urea reactions result in the direct formation of amorphous powders that are inerratic spherical and well dispersed.
2) Homogeneous precipitation method is adopted by using mutual compound of aluminium sulphate and aluminium nitrate to prepare the ultrafine α-Al2O3 powder. The configuration, morphology, particle size and surface area of the precursor are affected by the molar ratio of SO4- to NO3-. It is clear that the configuration of the precursor changes from colloid with bad filtered characteristics to spherical particles with good filterable performance as increasing molar ratio of SO42- to NO3-.
3) The precursor prepared by homogeneous pre- cipitation method using mutual compound of aluminium sulphate and aluminium nitrate with molar ratio of 40?60 as raw materials is spherical and has a narrow particle size distribution in the particle size range of 2-3 μm. The ultrafine alumina powder is spherical with uniform particle size distribution after the precursor is calcined.
References
[1] HUO Cai-xia, HE Li-jun. The preparation of nano-sized γ-Al2O3 powders by using urea as precipitating agent[J]. Journal of Gansu Lianhe University: Natural Science, 2004, 18(4): 45-47. (in Chinese)
[2] GU Feng, SHENG Yue, XU Chao, et al. The influence of polymerization degree of dispersing agent on the powder properties of nano alumina[J]. Journal of Functional Materials, 2005, 36(2): 318-320. (in Chinese)
[3] WANG Ya-juan, LI Chun-xi, WANG Zi-hao. Preparation of alumina nanometer particles by an ultrasonic precipitation method[J]. Journal of Beijing University of Chemical Technology, 2002, 29(4): 8-11. (in Chinese)
[4] ANANTHAPADMANABHAN P V, THIYAGARAJAN T K, SREEKUMAR K P, et al. Formation of nano-sized alumina by in-flight oxidation of aluminum powder in a thermal plasma reactor[J]. Scripta Materialia, 2004, 50: 143-147.
[5] LI Hui-yun, ZHANG Tian-sheng, YANG Nan. The preparation and application of nanometer-Al2O3[J]. Journal of Tianjin University of Light Industry, 2003, 18(4): 34-37. (in Chinese)
[6] WU Yi-quan, ZHANG Yu-feng, HUANG Xiao-xian, et al. Preparation of platelike nano alpha alumina particles[J]. Ceramics International, 2001, 27(2): 265-268. (in Chinese)
[7] TANG Hai-hong, JIAO Shu-hong, YANG Hong-ju, et al. Preparation and utilization of nanometer alumina[J]. China Powder Science and Technology, 2002, 8(6): 37-39. (in Chinese)
[8] ZHANG Yong-gang, YAN Fei. Preparation and application of nano-alumina[J]. Inorganic Chemicals Industry, 2001, 33(3): 19-22. (in Chinese)
[9] WU Zhi-hong. Preparation of nanoparticle alumina and its application in catalysis[J]. Industrial Catalysis, 2004, 12(2): 35-39. (in Chinese)
[10] MORINAGA K, TONKA T, NAKAGAWANA K, et al. Fabrication of fine α-alumina powders by thermal decomposition of ammonium aluminum carbonate hydroxide (AACH)[J]. Acta Mater, 2000, 48: 4735-4741.
[11] MA Chi-cheng, ZHOU Xue-xi, XU Xin, et al. Synthesis and thermal decomposition of ammonium aluminum carbonate hydroxide (AACH)[J]. Material Chemistry and Physics, 2001, 72(8): 374-379. (in Chinese)
[12] CHEN Cai-feng, CHEN Zhi-gang, HAO Chen, et al. Study on antiagglimeration of nanometer Al2O3 powder prepared by chemical precipitation[J]. Materials for Mechanical Engineering, 2000, 24(5): 26-28. (in Chinese)
[13] ZHANG Ai-fei, LIU Ji-ping. A new precipitation method for the preparation of alumina nanopowders[J]. Inorganic Chemicals Industry, 2003, 25(2): 27-28. (in Chinese)
[14] YANG Ye, WU Yu-cheng, LI Yong, et al. Preparation of ultrafine α-Al2O3 powder by thermal decomposition of AACH at low temperature[J]. The Chinese Journal of Process Engineering, 2002, 2(4): 325-329. (in Chinese)
[15] LI Jing, CHEN Shi-zhu. Preparation and characterization of nano-sized In2O3[J]. China Powder Science and Technology, 2003, 9(1): 33-35. (in Chinese)
[16] ADA K, SARIKAYA Y, ALEMDAROGLU T, et al. Thermal behaviour of alumina precursor obtained by the aluminium sulphate-urea reaction in boiling aqueous solution[J]. Ceramic International, 2003, 29(5): 513-518.
Foundation item: Project(5JJ3010) supported by the Natural Science Foundation of Hunan Province, China
Received date: 2007-05-08; Accepted date: 2007-07-16
Corresponding author: XIAO Jin, PhD; Tel: +86-731-8876474; E-mail: qi34qi@yahoo.com.cn
- Effect of anions on preparation of ultrafine α-Al2O3 powder


