网络首发时间: 2018-11-29 17:40
Na2CO3/ZnO复合添加对BaZr0.1Ce0.7Y0.2O3-δ质子导体陶瓷的影响Na2C03/Zn0复合添加对BaZr^e^Y^Og质子导体陶瓷的影响
昆明理工大学材料科学与工程学院
摘 要:
拟通过添加ZnO和Na2CO3来提高钇掺杂锆铈酸钡质子导体陶瓷的烧结性能和电导率,采用机械球磨混合结合高温常压烧结工艺制备了ZnO/Na2CO3复合的BaZr0.1Ce0.7Y0.2O3-δ(BZCY)质子导体陶瓷。通过X射线衍射仪(XRD)、扫描电子显微镜(SEM)、能谱(EDS)和电化学阻抗谱(EIS)对烧结陶瓷的物相、微观形貌、化学成分和电导率进行了测试表征。XRD结果显示ZnO/Na2CO3复合并没有改变BZCY的晶型。当烧结温度为1550℃保温6 h时,BZCY陶瓷的致密度和线性收缩率最大,分别达到96.15%和12.44%;添加1%ZnO(摩尔分数)后,烧结温度可降低至1350℃,陶瓷的致密度和线性收缩率分别达到96.87%和16.11%,可见,ZnO的添加可以大大提高BZCY的烧结性能。当添加10%(摩尔分数)Na2CO3后,陶瓷的晶粒尺寸约5~10μm,且没有引起BZCY的致密度和线性收缩率的显著降低。700℃时BZCY-1%ZnO-10%Na2CO3陶瓷的电导率可达1.225×10-2S·cm-1,而未添加Na2CO3的BZCY陶瓷的电导率仅为4.511×10-3 S·cm-1,电导率提高了约2.7倍,添加Na2CO3显著提高了BZCY-1%ZnO陶瓷的电导率。
关键词:
BaZrCeYO质子导体;烧结性能;电导率;NaCO/ZnO;
中图分类号: TM911.4;TQ174.75
作者简介:罗先游(1990-),男,贵州金沙人,硕士研究生,研究方向:功能陶瓷;E-mail:luoxianyou1990@163.com;;*孟彬,教授;电话:13238719568;E-mail:hitmengbin@163.com;
收稿日期:2018-09-08
基金:国家自然科学基金项目(51462018);云南省大学生创新创业训练计划项目(201710674203)资助;
Effects of Na2CO3/ZnO Addition on BaZr0.1Ce0.7Y0.2O3-δ Proton Conducting Ceramic
Luo Xianyou Zhao Mengyuan Xie Hao Bian Lingfeng Yang Xing Meng Bin
Faculty of Material Science and Engineering,Kunming University of Science and Technology
Abstract:
In order to improve the sintering performance and electrical conductivity of yttrium-doped Ba(ZrCe) O3-δ proton conducting ceramic,the ZnO and Na2 CO3 were added and the proton conducting ceramic of BaZr0.1Ce0.7Y0.2O3-δ(BZCY)+ZnO/Na2 C03 was prepared by mechanical ball milling combined with high temperature sintering in air atmosphere.The ciystalline structure,micro-morphology,chemical composition and electrical properties of the sintering ceramics were characterized by X-ray diffraction(XRD),scanning electron microscope(SEM),energy dispersive spectrometer(EDS) and electrochemical impedance spectroscopy(EIS),respectively.XRD showed that the addition of ZnO and Na2 C03 did not change the ciystal structure of BZCY.When the BZCY ceramics were sintered at 1550℃for 6 h,the relative density and linear shrinkage were optimal,i.e.,96.15% and 12.44%.The sintering temperature could be lowered to 1350 when the 1% ZnO was added,and the relative density and linear shrinkage could reach 96.87% and16.11%,respectively,and it could be seen that the addition of ZnO could greatly enhance the sintering properties of BZCY.The grains size of BZCY-1%ZnO ceramic with the Na2 C03 content of 10 mol% was about 5~10 μm and the addition of Na2 C03 did not decrease significantly in the relative density and linear shrinkage of BZCY.The Na2 CO3 addition was beneficial to improve the electrical conductivity of the BZCY-1%ZnO ceramic.At 700℃,the electrical conductivity of BZCY-1%ZnO-10%Na2 CO3 ceramic was optimal at 700℃,i.e.,1.225×10-2S·cm-1,while that of the BZCY-1%ZnO ceramic was only 4.511×10-3S·cm-1,the electrical conductivity increased by about 2.7 times.
Keyword:
BaZr0.1Ce0.7Y0.2O3-δ proton conductor; sintering performance; electrical conductivity; Na2CO3/ZnO;
Received: 2018-09-08
锆基或铈基电解质材料是固体氧化物燃料电池(SOFC)运用的主要候选材料,而受主掺杂的BaZrO3和BaCeO3是一类优异的质子导体电解质材料
在低价稀土元素掺杂的BaZrO3或BaCeO3中,通过在电解质的晶界处引入第二相来降低晶界阻抗已经引起了广泛的关注。晶界处第二相的存在会改变界面处的电荷分布,降低晶界势垒,从而提高晶界电导率。彭珍珍等
本文拟在添加ZnO烧结助剂的同时添加Na2CO3,利用机械球磨混合结合高温常压烧结制备了Na2CO3/ZnO复合的BaZr0.1 Ce0.7 Y0.2O3-δ(BZCY)质子导体陶瓷,并采用X射线衍射(XRD),扫描电镜(SEM),能谱(EDS)和电化学阻抗谱(EIS)对烧结陶瓷的物相、形貌、组成和电导率进行测试表征,研究了Na2CO3/ZnO的添加对BZCY陶瓷的烧结性能和电导率的影响。
1 实验
1.1 样品制备
首先,将分析纯碳酸钡(BaCO3:99%,Aladdin)、氧化锆(ZrO2:99%,Aladdin)、氧化铈(CeO2:99.95%,Aladdin)、氧化钇(Y2O3:99.9%,Aladdin)按既定摩尔比称量后,以无水乙醇为球磨介质,在氧化锆球磨罐中用氧化锆球进行球磨12 h (转速:350 r·min-1)。混合物经干燥、研磨、过筛后,于1300℃煅烧10 h,得到BaZr0.1Ce0.7Y0.2O3-δ(BZCY)前驱体粉末。之后,在BZCY前驱体粉末中加入x%ZnO (99.99%,Aladdin)(x=1,2,3),按照上述相同的球磨工艺,制得BZCY-ZnO系列混合粉末。然后在BZCY-1%ZnO混合粉末中加入y%Na2CO3(99.99%,Aladdin)(y=5,10),采用上述球磨工艺制备BZCY-1%ZnO-y%Na2CO3系列混合粉末。最后,将BZCY,BZCY-x%ZnO和BZ-CY-1%ZnO-y%Na2CO3系列混合粉末单轴干压成型,轴向压力420 MPa,保压30 min得到圆片型压片。然后,将BZCY生坯试样在1350,1450,1550℃下烧结保温6 h,制得BZCY陶瓷片;将BZCY-x%ZnO生坯试样在1350℃烧结保温6 h,制得BZ-CY-x%ZnO系列陶瓷片;将BZCY-1%ZnO-y%Na2CO3生坯试样在1300℃烧结保温4 h,制得BZCY-1%ZnO-Y%Na,CO3系列陶瓷片。
1.2 样品性能表征
采用游标卡尺(SLS 0-150MMX0.02)对烧结陶瓷片的直径5次测量求其平均值,然后计算烧结陶瓷试样的线收缩率。采用阿基米德排水法确定烧结陶瓷的实际密度,利用混合法则确定复合陶瓷试样的理论密度,然后计算得到相对密度。物相表征采用X射线衍射仪(XRD,D8 Advance)并结合Jade 6.0软件进行物相分析,比较前驱体粉末和烧结陶瓷的XRD图谱与标准PDF卡片的异同。采用扫描电子显微镜(SEM,JSM-840)对烧结陶瓷的断面形貌进行观察分析。
将烧结陶瓷试样的两平行表面进行研磨和抛光后,在陶瓷两表面上涂覆一层Ag浆料并连接出两根Ag丝,然后将陶瓷试片放置于110℃干燥箱中干燥30 min,最后将其置于加热炉中加热到800℃保温15 min得到涂覆有Ag浆料的陶瓷试片。电化学交流阻抗谱(EIS)的测试是采用阻抗分析仪(SP-300 BioLogic),测试温度范围为300~700℃,微扰电压为10 mV,测试频率范围为0.1 Hz~1 MHz,测试气氛为含饱和水蒸气的潮湿空气。在EIS测试之前,烧结陶瓷均在目标测试温度保温30 min以期达到热平衡。最后,利用ZSimpWin软件拟合阻抗谱并结合陶瓷试片的表面积和厚度计算其电导率。
2 结果与讨论
2.1 物相表征
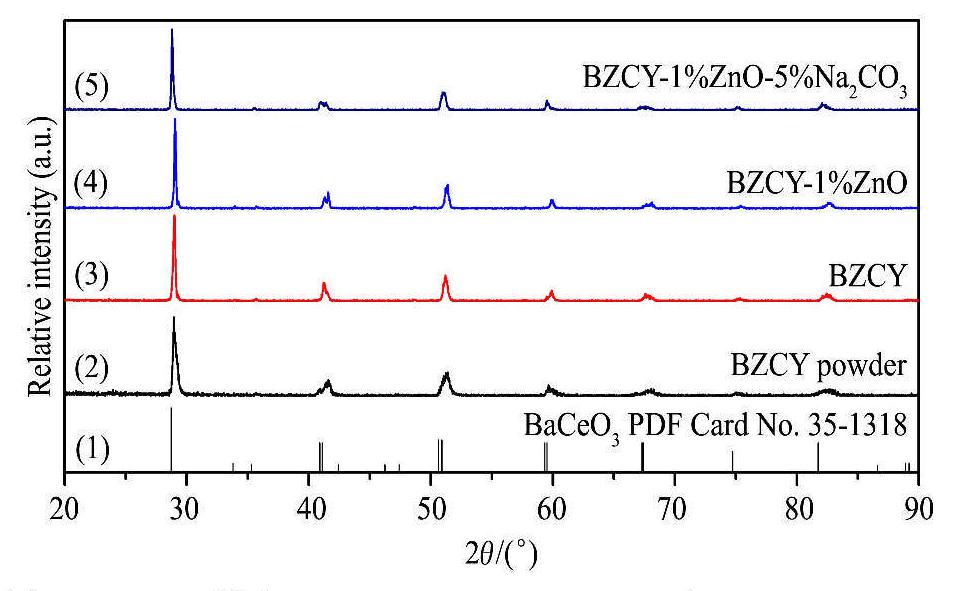
图1为BZCY前驱体粉末以及BZCY,BZCY-1%ZnO和BZCY-1%Zn0-5%Na2CO3烧结陶瓷的XRD图谱。从图1可以看出BZCY前驱体粉末、BZCY,BZCY-1%ZnO和BZCY-I%ZnO-5%Na2CO3烧结陶瓷的XRD图谱与BaCeO3标准图谱基本一致,没有明显的ZnO和Na2CO3对应的衍射峰存在,但与BaCeO3标准谱相比,BZCY前驱体粉末和BZCY烧结陶瓷的衍射峰略微向高角区方向偏移,这主要是由于Zr4+(0.072 nm)取代了部分Ce4+(0.078 nm)形成Ba(ZrCe)O3固溶体,从而导致Baa(ZrCe)O3的晶格缩小和衍射峰位偏移。当向BZCY中加入11%ZnO后,发现衍射峰进一步向高角度方向偏移(图1 (4)),这可能与Zn2+(0.074nm)部分固溶进入Ba(ZrCe) O3晶格中与Zr4+或Ce4+发生固溶取代所致。对于BZCY-1%ZnO和BZ-CY-1%ZnO-5%Na2CO3烧结陶瓷,ZnO的添加量较少(1%),低于XRD的检测极限,故XRD无法检测到ZnO的衍射峰。BZCY-1%ZnO-5%Na2CO3陶瓷的烧结工艺是1300℃烧结保温4 h,由于烧结温度较高,而Na,CO3的熔点仅为851℃,根据文献
图1 BZCY粉末,BZCY,BZCY-1%ZnO和BZCY-1%ZnC-5%Na2CO3烧结陶瓷的XRD图谱
Fig.1 XRD patterns of the BZY powder,BZCY,BZCY-1%ZnO and BZCY-1%ZnO-5%Na2CO3 sintered ceramics
2.2 烧结性能
2.2.1 烧结温度对BZCY致密度及线收缩率的影响
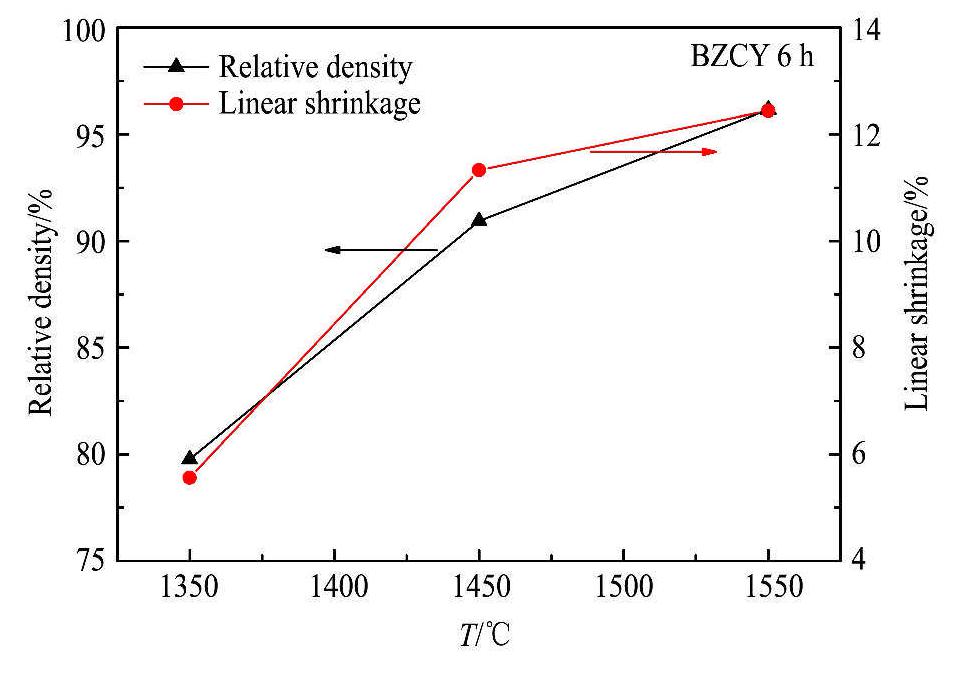
在陶瓷烧结过程中合理控制烧结温度和保温时间是很必要的,合适的烧结工艺可以得到性能优异的烧结陶瓷。当保温时间均为6 h时,在不同烧结温度下的BZCY致密度和线收缩率如图2所示。当烧结温度从1350℃升高到1550℃,烧结陶瓷的致密度和线收缩率均呈增大趋势,BZCY的致密度从79.77%提高到96.15%,线收缩率从5.56%提高到12.44%。可见,在合适的烧结温度范围内,烧结温度越高,越有利于陶瓷的烧结致密化。
2.2.2 ZnO添加量对BZCY致密度及线收缩率的影响
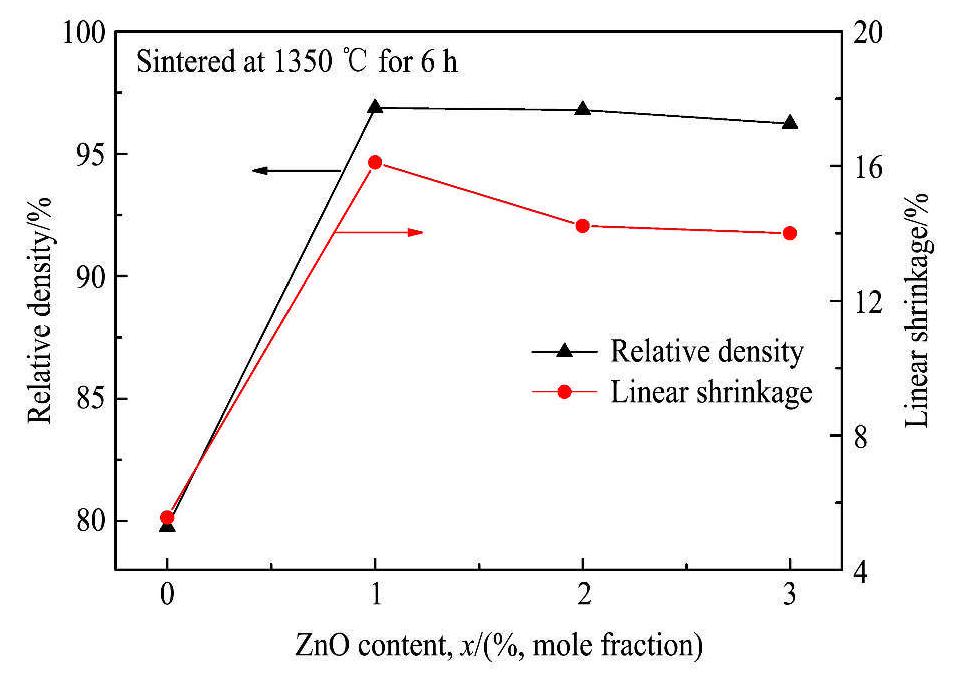
在陶瓷烧结过程中,添加合适的烧结助剂可以提高陶瓷的烧结性能,降低烧结温度、缩短保温时间。图3显示了当烧结温度均为1350℃、保温6 h时,加入ZnO做烧结助剂后BZCY陶瓷的致密度和线收缩率随ZnO添加量的变化情况。随着ZnO添加量从0增加到3%,烧结陶瓷的致密度从79.77%升高到96.87%,然后缓慢降低到96.23%;线收缩率从5.56%升高到16.11%,然后缓慢降低到14%。可见,ZnO能显著提高BZCY陶瓷的烧结性能,降低烧结温度、缩短保温时间,这主要是由于在陶瓷烧结过程中,ZnO可以和Ba的氧化物形成低共熔物
图2 烧结温度对BZCY致密度及线收缩率的影响
Fig.2 Effects of sintering temperature on relative density and linear shrinkage of BZCY
图3 ZnO添加量对BZCY的致密度及线性收缩率的影响
Fig.3 Effects of the ZnO content on relative density and linear shrinkage of BZCY
2.2.3 添加Na2CO3对BZCY-ZnO陶瓷的致密度及线收缩率的影响
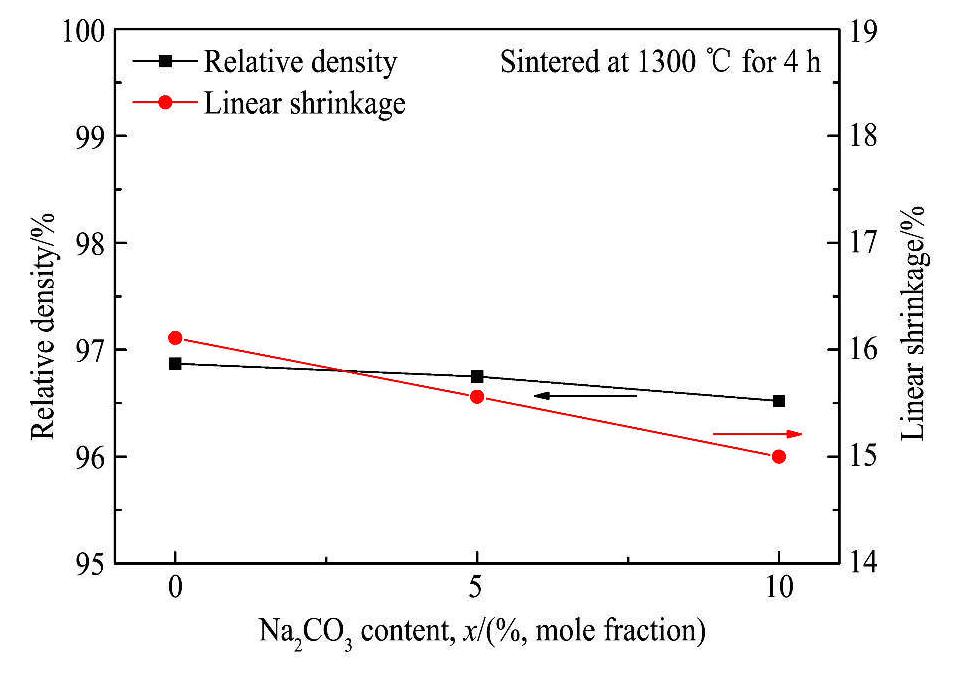
图4为添加不同含量Na2CO3后BZCY-1%ZnO在1300℃烧结保温4 h后的致密度及线收缩率的变化情况。当Na2CO3的添加量为5%时,陶瓷的致密度和线性收缩率为96.75%和15.56%;当Na2CO3的添加量继续增加到10%时,陶瓷的致密度和线性收缩率分别为96.52%和15%。与未添加Na2CO3的BZCY-1%ZnO样品相比,加入Na2CO3后,将烧结温度由1350℃降低至1300℃后,没有引起致密度和线性收缩率的显著降低。因此添加Na2CO3可以降低BZCY-1%ZnO的烧结温度、缩短其保温时间,从而提高陶瓷的烧结性能。这主要是由于Na2CC3的熔点为851℃,在较高的烧结温度下,Na2CO3呈现为液相,继而利用液相烧结机制来促进陶瓷烧结。
2.3 微观形貌
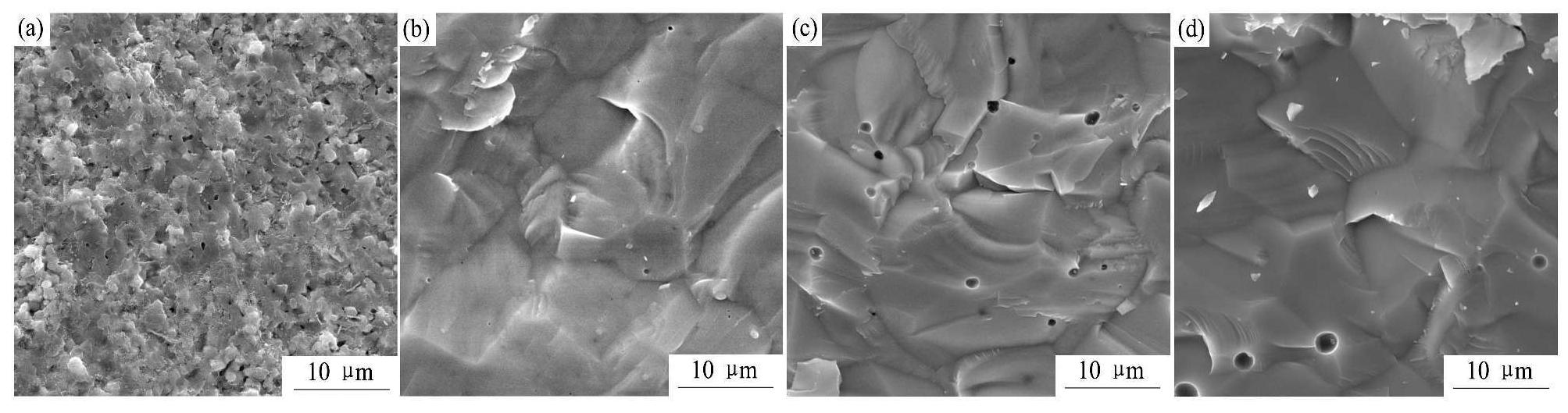
2.3.1 不同ZnO添加量的BZCY烧结陶瓷的断面形貌
不同ZnO添加量的BZCY烧结陶瓷的断面形貌如图5所示。图5(a)是烧结温度为1450℃、保温6 h的BZCY烧结陶瓷的断面形貌,BZCY的晶粒大小均匀,约为1.0~1.5μm,其结构疏松,存在许多小孔洞,不够致密。图5 (b~d)分别表示ZnO添加量为1%,2%,3%的BZCY烧结陶瓷的断面形貌,当ZnO添加量为1%时,BZCY-1%ZnO烧结陶瓷的孔洞减少且孔洞尺寸降低,结构较致密;当ZnO添加量增加到2%和3%时,烧结陶瓷的孔洞少但孔洞尺寸较大,致密性反而降低。这与图3所示的ZnO添加量对BZCY的致密度及线收缩率的影响变化趋势是致的。
图4 Na2CO3添加量对BZCY-1%ZnO的致密度及线收缩率的影响
Fig.4 Effects of Na2CO3 content on density and linear shrinkage of BZCY-1%ZnO
图5 不同ZnO添加量的BZCY烧结陶瓷的断面形貌
Fig.5 Fracture surface SEM images of BZCY with different content of ZnO
(a) BZCY (1450℃/6 h);(b) BZCY-1%ZnO(1350℃/6 h);(c) BZCY-2%ZnO (1350℃/6 h);(d) BZCY-3%ZnO(1350℃/6 h)
2.3.2 不同Na2CO3添
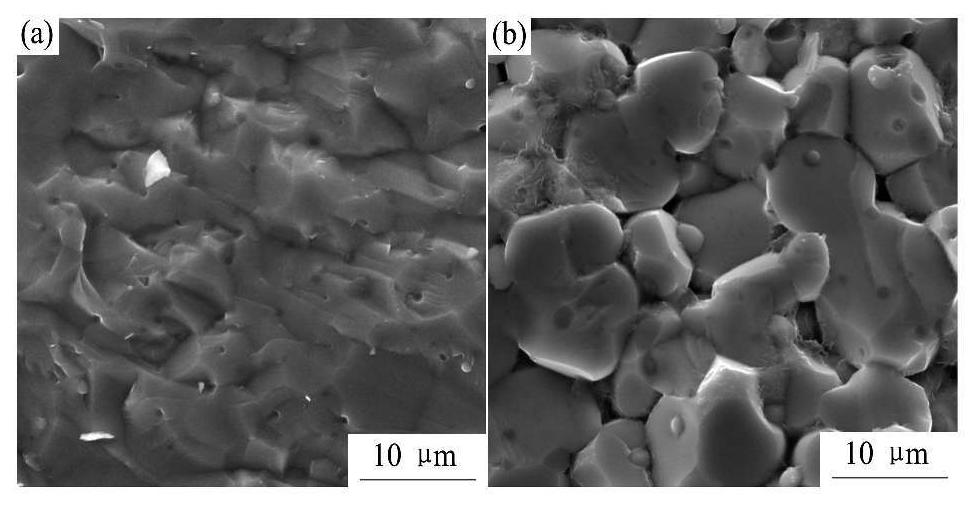
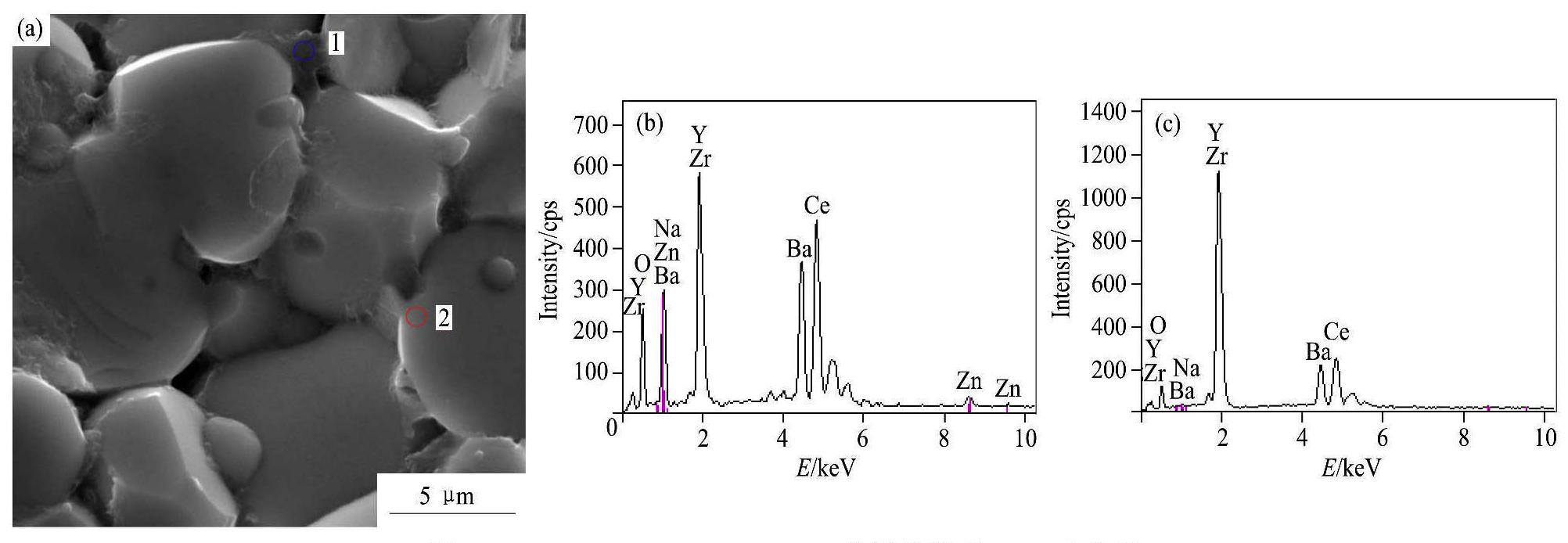
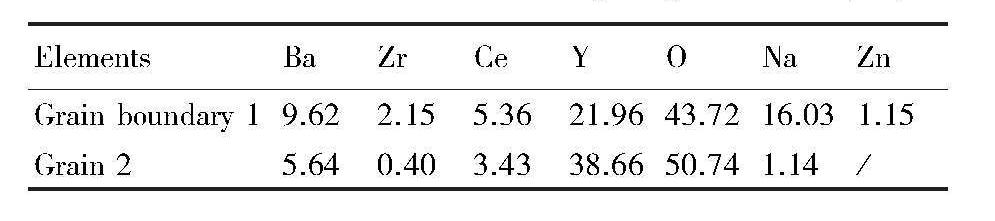
加量的BZCY-1%ZnO烧结陶瓷的断面形貌及化学成分添加不同含量Na2CO3的BZCY-1%ZnO烧结陶瓷的断面形貌如图6所示。图6(a)为添加5%NaCO3的BZCY-1%ZnO的断面形貌,可见有少许小孔存在,结构较致密。图6(b)为添加10%Na2CO3的BZCY-1%ZnO的断面形貌,可见晶粒间有明显大孔隙,晶粒尺寸约5~10μm,相比于添加5%Na2CO3的BZCY-1%ZnO烧结陶瓷,致密性稍差。BZCY-1%ZnO-10%Na2CO3烧结陶瓷的EDS点分析如图7所示,通过能谱(EDS)点分析可以看出,晶界位置1的元素包括:Ba,Ce,Zr,Y,Na和Zn;晶粒边界位置2的元素包括:Ba,Ce,Zr,Y和Na。晶界位置1和晶粒边界位置2元素的原子分数如表1所示,可见,晶界位置1和晶粒边界位置2都有Na元素存在,晶界位置1还存在Zn元素,但并未发现C元素存在,说明Na2CO3在高温烧结过程中发生分解。根据文献报道,在BaZrO3陶瓷的烧结过程中,ZnO主要聚集在晶界区,且Zn2+(0.074 nm)可固溶进ABO3钙钛矿结构中的B位
图6 不同Na2CO3添加量的BZCY-1%ZnO烧结陶瓷的断面形貌
Fig.6 Fracture surface SEM images of BZCY-1%ZnO with dif-ferent content of Na2CO3
(a) BZCY-1%ZnO-5%Na2CO3(1300℃4 h);(b) BZCY-1%ZnO-10%Na2CO3(1300℃/4 h)
图7 BZCY-1%ZnO-10%Na2CO3烧结陶瓷的PDS点分析
Fig.7 EDS point analysis of BZCY-1%ZnO-10%Na2CO3 sintered ceramic
(a) Micrograph;(b) EDS of Grain boundary 1;(c) EDS of Grain 2
表1 BZCY-1%ZnO-10%Na2 CO3陶瓷EDS分析中晶界位置1和晶粒边界位置2各元素原子分数 下载原图
Table 1 Atomic fraction of each element at Grain boundary 1 and Grain 2 in EDS analysis of BZCY-1%ZnO-10%Na2 CO3 ceramics (%)
2.4 电化学交流阻抗谱和电导率
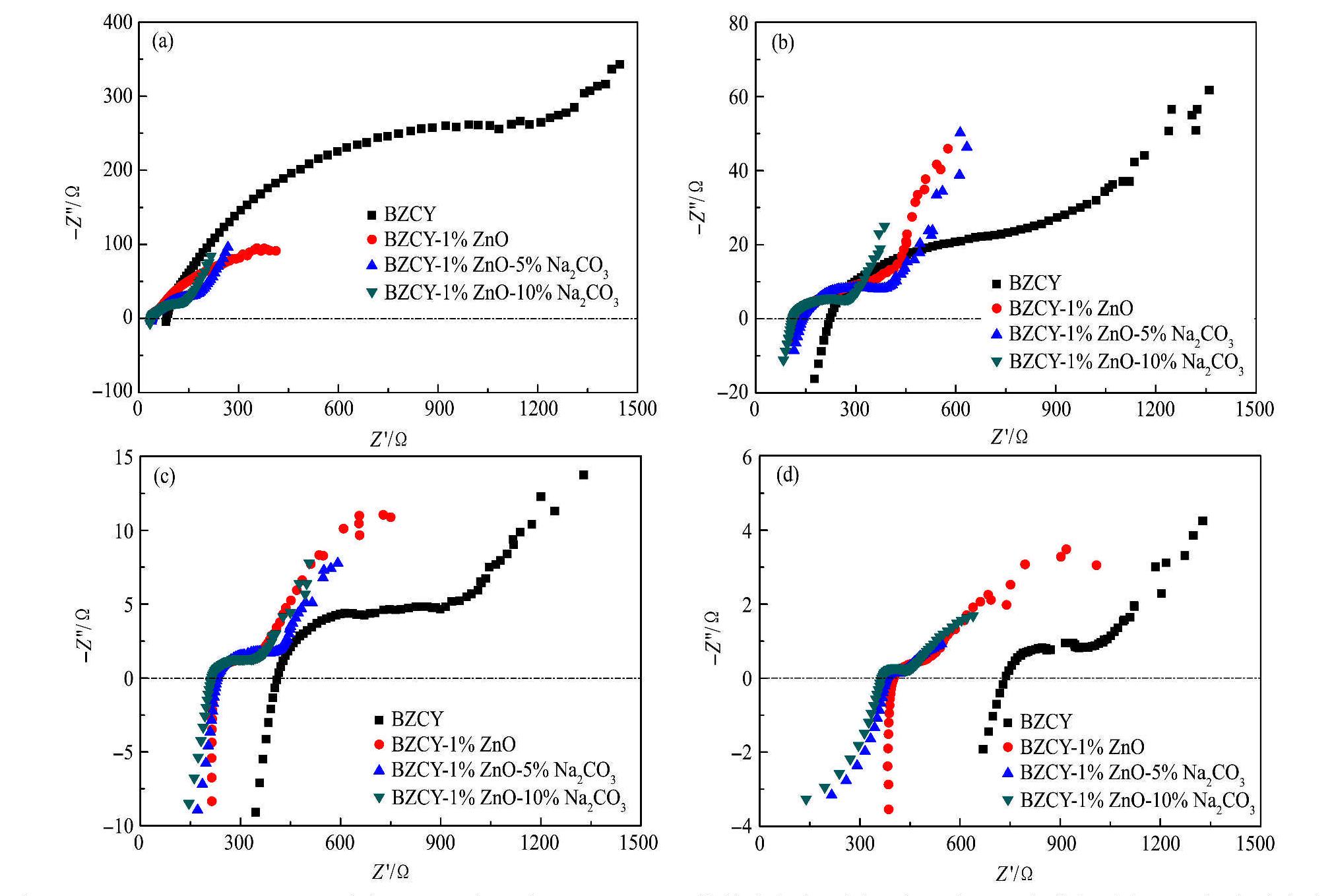
电化学交流阻抗谱测试是在含饱和水蒸气的潮湿空气中进行的,BZCY,BZCY-1%ZnO和不同Na2CO3含量的BZCY-1%ZnO在400,500,600和700℃下的交流阻抗谱如图8所示。根据交流阻抗的基本理论
在400,500,600和700℃各测试温度下,随着测试温度的升高,第二个半圆弧与实轴的截距值逐渐减小,即陶瓷总的欧姆阻抗逐渐减小。在400,500和600℃测试温度点,BZCY的欧姆阻抗均最大,BZCY-1%ZnO-10%Na2CO3的欧姆阻抗均最小。测试温度为700℃时,BZCY的欧姆阻抗约为13.64Ω,BZCY-1%ZnO-10%Na2CO3的欧姆阻抗约为5.77Ω。可见,由于Na2CO3的加入,可以降低BZCY的阻抗值,且Na2CO3的添加量为10%时阻抗值最低。
图8 BZCY,BZCY-1%/nO和不同Na2CO3含量的BZCY-l%ZnO烧结陶瓷在不同温度下含饱和水蒸气的潮湿空气中的交流阻抗谱
Fig.8 AC impedance plots of BZCY,BZCY-l%ZnO and BZCY-l%ZnO with different Na2CO3 contents at different temperatures in moist air containing saturated water vapor
(a) 400℃;(b) 500℃;(c) 600℃;(d) 700℃
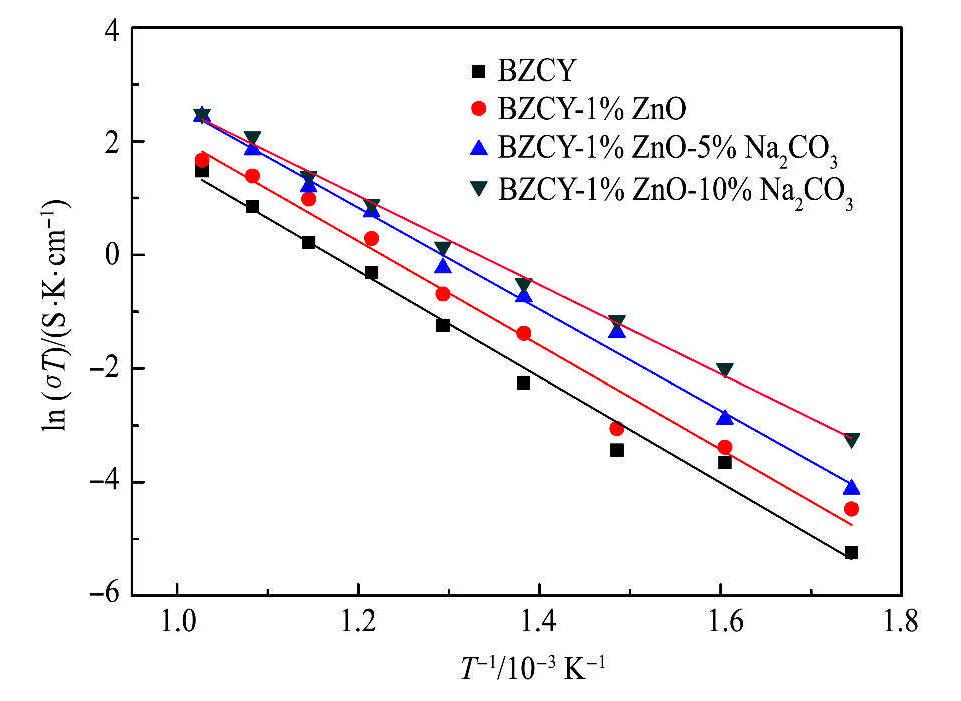
BZCY,BZCY-1%ZnO和不同Na2CO3添加量的BZCY-1%ZnO在含饱和水蒸气的潮湿空气中的Arrhenius图如图9所示。在300~700℃的测试温度范围内,BZCY,BZCY-1%ZnO和不同Na2CO3添加量的BZCY-1%ZnO烧结陶瓷的电导率均随测试温度的升高而增大。BZCY,BZCY-1%ZnO和BZCY-1%ZnO-5%Na2CO3陶瓷的Arrhenius图的拟合曲线大致平行,表明三者的导电机制一致,而BZCY-1%ZnO-10%Na2CO3陶瓷的Arrhenius图的拟合曲线的斜率与前三者相比发生了改变,说明其导电激活能发生了变化。
在300-700℃测试温度范围内的各测试温度点,BZCY-1%ZnO-10%Na2CO3烧结陶瓷的电导率值均最高,而BZCY烧结陶瓷的电导率值均最小。在700℃时,BZCY-%ZnO-10%Na2CO3烧结陶瓷的电导率可达1.225×10-2 S·cm-1,而BZCY烧结陶瓷的电导率仅为4.551×10-3 S·cm-1。可见Na2CO3的添加可以提高BZCY-1%ZnO-10%Na2CO3陶瓷的电导率,这可能是由于分布于晶界处的Na2CO3,改变了晶界的电荷分布状态,降低了晶界势垒,从而降低了晶界阻抗,提高了电导率。此外,Na和Zn的固溶也会增加Ba空位和O空位浓度,从而可以提高质子电导率。
图9 BZCY,BZCY-l%ZnO和不同Na2CO3添加量的BZCY1%ZnO在含饱和水蒸气的潮湿空气中的电导率
Fig.9 Conductivities of BZCY,BZCY-1%ZnO and BZCY-1%ZnO in different content of Na2CO3 under the moist air containing saturated water vapor
3 结论
本研究采用机械球磨混合结合高温常压烧结制备了BZCY-ZnO-Na2CO3系列烧结陶瓷。当烧结温度为1550℃、保温6 h时,BZCY烧结陶瓷的致密度和线性收缩率分别达到96.15%和12.44%。当烧结温度为1450℃、保温6 h时,BZCY烧结陶瓷的晶粒大小均匀,尺寸约1.0~1.5当添加1%ZnO做烧结助剂后,烧结温度降低至1350℃并保温6 h时,BZCY-1%ZnO陶瓷的致密度和线性收缩率也可达96.87%和16.11%。
在测试温度范围为300~700℃内,添加Na2CO3可以提高BZCY-1%ZnO烧结陶瓷的电导率。当Na2CO3的添加量为10%时,BZCY-1%ZnO-10%Na2CO3陶瓷的电导率在各测试温度点均最高,当测试温度为700℃时,BZCY-1%ZnO-10%Na2CO3陶瓷的电导率可达1.225×10-2 S·cm-1,而未添加Na2CO3的BZCY陶瓷的电导率仅为4.511×10-3 S·cm-1。
参考文献