
Low temperature preparation of nano TiO2 and its application as antibacterial agents
Deng Hua(邓 桦) 1, 2, K. Cheuk3, ZHENG Wei-ning(郑卫宁) 2,
WEN Chen(文 晨)2, XIAO Chang-fa(肖长发) 2
1. School of Textiles, Tianjin Polytechnic University, Tianjin 300160, China;
2. Tianjin Municipal Key Lab of Fiber Modification and Functional Fiber, Tianjin Polytechnic University,
Tianjin 300160, China;
3. Institute of Textiles & Clothing, The Hong Kong Polytechnic University, Hong Kong, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
A low temperature preparation of nano TiO2 using sol-gel method was proposed. The antibacterial properties of the treatment solution and the treated cotton fabrics obtained via a dip-padding process were evaluated. XRD pattern shows that the nano TiO2 produced is an anatase phase. Aqueous nano-dispersion of the nano TiO2 exhibites positive results as an antibacterial finishing agent for cotton fabrics. The treatment solution possesses antibacterial rates of over 92% and 88.9% against Escherichia coli and Bacillus subtilis, respectively. The treated fabrics are slightly downgraded but still maintained over 89% and 83% of reduction towards the same bacteria. After washing for 50 times, the antibacterial performances of the treated fabric still remains at a relatively high level, indicating the durable characteristic of nano TiO2 treatment.
Key words:
nano- titania; crystal grain; antibacterial properties; cotton fabric;
1 Introduction
As a new material, nanosized TiO2 is of great interests of many scientists in the recent years. Its small size and large specific surface area allow for certain unique and unusual physico-chemical properties [1]. Moreover, due to its high chemical stability, non-toxicity and good heat resistance, it is highly promising to be used in electronic, photocatalysis, water purification, as well as antibacterial and self-cleaning materials [2-4]. Nano TiO2 has three crystal structures: anatase, rutile, and brukide. Anatase has higher activity and is particularly suitable to be used as as an antibacterial material[5-6].
Among the various ways of nanosized TiO2 synthesis, the sol-gel method is the most commonly used [7]. Precursor such as tetrabutyltitanate is hydrolyzed to develop a sol and the product is formed firstly via a gelling process after drying. Nanosized TiO2 powder or film can thus be obtained after high temperature annealing [8-10]. The application of nano TiO2 in the processing of textiles is typically achieved by doping powdery nano TiO2 into synthetic filament fibers [11-12]; or by dispersing powdery nano TiO2 to form aqueous dispersion designed for fabric/yarn finishing [13]. However, there are some drawbacks of using TiO2 powder for textile finishing, such as aggregation of nano particles, non-uniformity of the dispersion, and instability of the finishing agent. Moreover, the interaction between the nano TiO2 and the fabric substrate is relatively weak and therefore the durability would normally be interfered. These issues restrict the use of nano TiO2 to textile applications partially.
In this study, the synthesis of anatase crystal type of nano TiO2 by sol-gel method at low temperature (50 ℃) was reported. Further more, the aqueous solution (a finishing agent for textiles) containing nano TiO2 was prepared through liquid/liquid mixing. This process can avoid high temperature annealing and overcome above mentioned issues such as aggregation of nano powder. Finally, its application in antibacterial finishing of textiles is evaluated.
2 Experimental
2.1 Preparation of nano- titania
Tetrabutylorthotitanate (25 g) and ethanol (2 g) were evenly mixed in a beaker. Then the mixture was dripped into 75 mL aqueous acid (inorganic acid, 1%, mass fraction) and allowed for mixing for 4 h with stirring at 50 ℃. Under stirring, the prepared TiO2 sol was mixed with dispersant FK (from CTA-Tex Chemicals Co) and water to form fabric finishing agents of 1%, 5%, and 10% concentrations of TiO2, respectively.
2.2 Measurement
The shape and size of nano-titania particles were investigated under a transmission electron microscope (TEM; JEOL JEM-2011) operated at 200 kV. In short, a few milliliters of prepared TiO2 sol was dropped onto clean copper mesh grids and allow natural drying at room temperature. The crystallinity of nano-titania was analyzed using X-ray diffraction spectroscopy (XRD; Bruker D8 Discover X-ray Diffractometer with GADDS, Cu Kα) operating at 40 kV.
2.3 Fabric treatment
A scoured woven cotton fabric was used in the experiment. The cleaned fabrics were dipped in nano TiO2 finishing agents for 2 min, dip-pad, wet picked up about 80%, set for 2 min, rinsed by water, and dried at 80 ℃ for 5 min and 100 ℃ for 3 min.
2.4 Antibacterial activity
The antibacterial activities of TiO2 for the TiO2 finishing agents and the treated fabrics were evaluated quantitatively according to Dow Corning Shake Flask Test Method [14]. The test determines the reduction in the number of bacterial cells after placing the sample in a shaking flask for 24 h. Escherichia coli and Bacillus subtilis, Gram-negative and Gram-positive bacteria respectively, were chosen as the testing bacteria. A typical protocol was as follows: (0.75±0.05) g of sample fabric was dipped into a flask containing 70 mL of 0.03 mol/L PBS (phosphate buffer saline) culture solution with a cell concentration of (1-5)×106/mL. The flask was then shaken at 250 r/min on a rotary shaker at 37 ℃ for 24 h. After shaking, 1 mL of the testing solution was extracted, diluted and spread onto an agar plate. After incubation at 37 ℃ for 24 h, the number of colonies formed on the agar plate was counted and the results were expressed as mean colony forming units per milliliter, after incubation. Antibacterial efficacy was determined on the basis of duplicates test results. Percentage bacterial reduction was calculated according to the following equation:
![]()
where R is the percentage of bacterial reduction; B and A are the average number of live bacterial cells per milliliter in the flask of the control and nano-titania finishing agent or treated fabrics, respectively.
Wash fastness of the treated fabrics was accessed according to AATCC 61—2001 standard methods [15].
3 Results and discussion3.1 Characterization of titania particles
Drop a few milliliters of prepared TiO2 sol on a copper mesh grid and allow natural drying at room temperature. Fig.1 shows TEM micrograph of the prepared TiO2 particles. It can be seen that the particles are spherical in shape and have a uniform particle size distribution in diameters of approximate 8 nm. An insert in the figure shows the diffraction pattern of the sample. The diffusion rings indicate a fine nano-crystalline structure. As given in Fig.2, XRD pattern of the TiO2 particles dried at room temperature exhibits two peaks at 25.4? and 37.8?, which rationally corresponds to TiO2 (101) and TiO2 (004) diffractions of the anatase structure, respectively. When combined with the TEM analysis, it can be confirmed that the nano TiO2 synthesized under the low temperature condition is crystalline and its anatase phase is obtained.
3.2 Antibacterial activity of nano-titania finishing agent
The antibacterial performance of the nanosized TiO2 are summarized in Table 1.
The nano TiO2 finishing agents chearly demonstrate an ability to inhibit the growth of bacteria. In comparison with the control, 1% of TiO2 in the solution can reduce
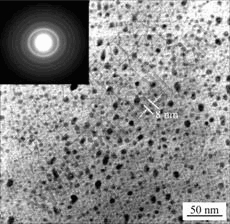
Fig.1 TEM image of prepared titanium dioxide
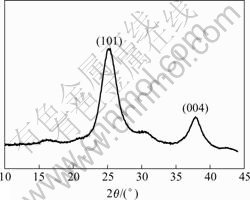
Fig.2 XRD pattern of TiO2 sample dried at room temperature
Table 1 Antibacterial activities of nano TiO2 solution
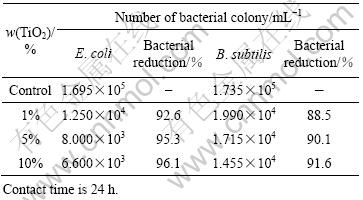
over 88% of the total bacteria population, while 5% or above of TiO2 can even reduce over 90%. As nano TiO2 is photocatalytically active, certain light energy is absorbed by nano TiO2 in the solutions and then transferred to some other forms of excited active species. In fact, TiO2 contains energy bands, which are discrete and there thus exists a gap (about 3.2 eV) between the valance band and the conduction band. When TiO2 is irradiated by UV rays or sunlight, a number of charge-hole pairs will be created in these bands. The holes (h+) are able to react with water molecules or hydroxyl groups, resulting in hydroxyl radicals, which are strong oxidants to organic molecules or bacterial cells [16]. At the same time, the electrons can reduce the oxygen molecules in air to produce active anion radicals ![]() . Because of their instability, the anion radicals formed are highly reactive. They may diminish the bacterial cell metabolism as well as the reproduction ability as a result of undergoing further reactions with the cell components in the bacteria. Consequently, both the holes and the charges generated in the process under light source are believed to be the active components in the antibacterial mechanism [17]. Their formation reactions are proposed as follows:
. Because of their instability, the anion radicals formed are highly reactive. They may diminish the bacterial cell metabolism as well as the reproduction ability as a result of undergoing further reactions with the cell components in the bacteria. Consequently, both the holes and the charges generated in the process under light source are believed to be the active components in the antibacterial mechanism [17]. Their formation reactions are proposed as follows:
TiO2+hν→TiO2+h++e- (1)
h++H2O→?OH+H+ (2)
e-+ O2→![]() (3)
(3)
The results also reveal that the antibacterial activities increase with the increase of concentration of TiO2 in the solutions. However, too high TiO2 concentration is not recommended as nano TiO2 particles tend to aggregate in solution, thus making the system less stable for storage.
3.3 Antibacterial effects of treated fabric
When cotton fabrics are treated with the nano TiO2 treatment solutions, the treated fabrics also possess very good antibacterial ability similar to that of the solutions. As shown in Table 2, the resistances to Escherichia coli and Bacillus subtilis reach about 90% and 84%, respectively, for the fabrics dealt with 1% of TiO2. The bacterial reduction efficiency is directly proportional to the concentration of the nano TiO2 solution regardless of the bacteria used here, achieving as high as 94% and 89% bacterial reductions (10% TiO2 treatment), respectively. As the amount of the metal oxide increases, the active ingredients produced from the photocatalytic process increases, hence contributing to the antibacterial performance.
Table 2 Antibacterial activities of treated cotton fabric
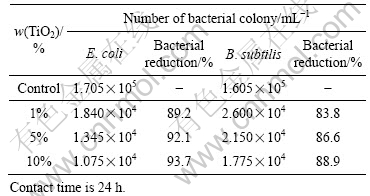
From the above results, it is generally agreed that the nano TiO2 has stronger effect on Escherichia coli than on Bacillus subtilis. The reason may attribute to the cell wall difference between the two distant bacteria, in which Escherichia coli has thinner and slack cell walls, while Bacillus subtilis has thicker and denser cell wall. This order of precedence appears to be reasonable if it is assumed that the primary step in the photocatalytic decomposition consists of attacks by the anion radicals on the cell wall, leading to the formation of punctures and finally death of cell [6].
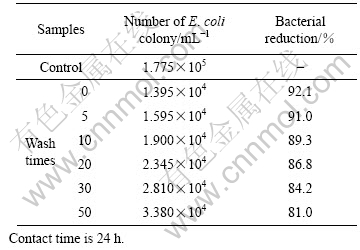
3.4 Wash fastness of antibacterial fabric
Wash fastness is an important aspect for durability assessment of the antibacterial fabrics. In this study, Escherichia coli strains were used as the model bacterium to determine the change of the antibacterial effect. Table 3 shows the antibacterial results of the treated fabrics after accumulated wash cycles. With the increase of the wash cycle, the antibacterial activity of treated fabrics gradually declines, but the extent is not significant. After 20 washes, the bacterial resistance drops only 5%, while the drop is just 11% when the washing gets to as many as 50 cycles. Moreover, the bacterial reduction effectiveness still maintains at 81%, indicating that the antibacterial coating on the fabrics is not much affected by the laundry washing. Such excellent adhesion properties between the TiO2 coating and the cotton substrate can be explained as follows: nano TiO2 particles are small enough to enter into the gaps of the cotton fibers, resulting in stronger interaction with the fibers.
Table 3 Durability of antibacterial fabric(5% TiO2)
4 Conclusions
1) Crystalline nano TiO2 can be prepared using the sol-gel process at low temperature without annealing.
2) Nano TiO2 prepared by this method is in anatase phase and the particle size is about 8 nm. The agents in different concentrations give good antibacterial performance against Escherichia coli and Bacillus subtilis regardless of the stage of the agent before or after coating on fabrics.
3) The antibacterial efficiencies of the treated cotton fabrics are 93.7% for Escherichia coli and 88.9% for Bacillus subtilis, respectively. Furthermore, the antibacterial properties of the finishing agent and the fabrics are directly proportional to the concentration of the nano TiO2 solution.
4) The wash fastness of the treated fabrics is also superior, in which the bacterial reduction against Escherichia coli remains to be 81% of the original after 50 wash cycles. This technique provides a new way to industrialize the production of antibacterial self-cleaning textiles.
References[1] ZHANG Li-de, MOU Ji-mei. Nano-materials and nano-structure [M]. Beijing: Science Press, 2002: 68-93. (in Chinese)
[2] YEON S M, KYUN K D, JIN I K. Electrospun TiO2 electrodes for dye-sensitized solar cells [J]. Nanotechnology, 2004, 15(8): 1861-1865.
[3] Wauthoz P, Ruwet M, Machej T, Grange P. Influence of the preparation method on the vanadia/titania/silica catalysts in selective catalytic reduction of nitric oxide with ammonia [J]. Applied Catalysis, 1999, 69(1): 149-167.
[4] LEE C K, KIM J K, LEE J H. Preparation and characterization of peroxo titanic acid solution using TiCl3 [J]. J Sol Gel Sci Technol, 2004, 31(1/3): 37-42.
[5] GE Lei, XU Ming-xia, SUN Ming. Low temperature preparation and photocatalytic properties of Nano TiO2 thin films [J]. Journal of the Chinese Ceramic Society, 2006, 34(5): 536-540. (in Chinese)
[6] XU Wei-guo, CHEN An-min, ZHANG Qiang. Preparation of TiO2 thin film and its antibacterial activity [J]. Journal of Wuhan University of Technology: Mater Sci Ed, 2004,19(1): 26-28.
[7] DING X Z, LIU X H. Synthesis and microstructure control of nanocrystalline titania powders via a sol-gel process [J]. Materials Science and Engineering A, 1997, A224(1/2): 210-215.
[8] LEE Y C, JUNG Y J. Preparation of TiO2 powder by modified two-stage hydrolysis [J]. Journal of Sol-gel Science and Technology, 2004, 30(1): 21-28.
[9] KHOLMANOV I N, BARBORINI E, VINATI S. The influence of the precursor clusters on the structural and morphological evolution of nanostructured TiO2 under thermal annealing [J]. Nanotechnology, 2003, 14(5): 1168-1173.
[10] REN Da-sen, CUI Xiao-li, SHEN Jie. Study on the superhydrophilicity of SiO2-TiO2 thin films prepared by sol-gel method [J]. Journal of Sol-gel Science and Technology, 2004, 29(3): 131-136.
[11] Teng Cui-qing, Yu Mu-huo. Preparation and property of poly (ethylene terephthalate) fibers providing ultraviolet radiation protection [J]. Journal of Applied Polymer Science, 2003, 88(2): 1180-1185.
[12] QIAN Lei. Nanotechnology in textiles [J]. AATCC Review, 2004, 4(5): 14-16.
[13] Yang Hong-ying, ZHU Su-kang, PAN Ning. Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme [J]. Journal of Applied Polymer Science, 2004, 92(3): 3201-3210.
[14] YALPANI M, JOHNSON F, ROBINSON L E. Advances in chitin and chitosan[M]. New York: Elsevier Applied Science, 1995: 543-561.
[15] AATCC Test Method 61—2003[M]. Colorfastness to Laundering, Home and Commercial: Accelerated, 2003, 90-94.
[16] FUJISHIMA A, RAO N, TATA A, et al. Titanium dioxide photocatalysis [J]. Photochem Photobid C: Photochem Rev, 2000, 1: 13-21.
[17] PIN-CHING M, SMDINSKI S, BLAKE D, et al. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism [J]. Appl Environ Microbiol, 1999, 65(10): 4094-4098.
Foundation item: Project (06YFJMJC02700) supported by Municipal Natural Science Foundation of Tianjin, China
Corresponding author: Deng Hua; Tel: +86-22-24528370; E-mail: dengh2@126.com


