
Lead desorption from modified spent grain
LI Qing-zhu(李青竹), CHAI Li-yuan(柴立元), ZHAO Jing(赵 静),
YANG Zhi-hui(杨志辉), WANG Qing-wei(王庆伟)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 25 August 2008; accepted 8 November 2008
Abstract:
Lead-loaded modified spent grain regenerated by desorption process was investigated. HCl, H2SO4, H3PO4, NaOH, NaCl and ultrapure water were chosen as desorption agents to treat lead-loaded modified spent grain for 30 min. The structures and components of regenerated modified spent grain before and after adsorbing Pb(Ⅱ) were investigated using scanning electron microscopy(SEM), energy-dispersive analysis of X-ray(EDAX) and Fourier transform infrared spectrometry(FTIR). The results indicate that lead-loaded modified spent grain treated in 0.1 mol/L HCl exhibits higher elution efficiency (86.44%) as compared with other agents. The enrichment of carboxyl and hydroxyl groups susceptible to combine with Pb(Ⅱ) are observed in the regenerated modified spent grain, which may result in high re-absorption efficiency of Pb(Ⅱ). Moreover, C—Cl, N—H, C—N and O—H (polysaccharides) also play an crucial role in Pb(Ⅱ) binding to regenerated modified spent grain.
Key words:
Pb(Ⅱ); spent grain; desorption; re-adsorption; mechanism;
1 Introduction
The removal of toxic heavy metal ions from wastewater is of great importance from an environmental point of view. Adsorption has been demonstrated to outperform other techniques in treating wastewater due to its low cost, high efficiency, being simple and easy to perform, and being insensitive to toxic substances[1]. The adsorption of metal ions by industrial waste products [2-3] and other natural materials[4-7] has potential for industrial use. Modified spent grain(MSG), as a new adsorbent, is an appealing alternative for removing heavy metals from wastewater as it has a large specific surface area and small size, with hollow and layered structures containing many functional groups such as carboxyl, hydroxyl and amino that are responsible for the binding of metal ions.
If the adsorption process is used as an alternative in wastewater treatment, the desorption and regeneration of adsorbent may be crucially important to keep low processing costs and open the possibility to recover the extracted metal(s) from the liquid phase. Desorption process yields metals in a concentrated form, which facilitates disposal and restore adsorbents for effective reuse. In recent years, new techniques for adsorbent regeneration have attracted considerable interest. Regeneration procedures include thermal treatment[8], chemical extraction[9-10], bio-regeneration[11], super- critical regeneration[12], microwave irradiation[13] and ultrasonic regeneration[14-15]. Chemical extraction using NaOH, HCl and HNO3 is most often applied to regenerating exhausted adsorbent. According to Refs.[16-18], HCl was used to desorb lead, and the metal recovery percentage ranged from 76.92% to 99.60%. But there is little study about the mechanism of lead desorption and re-adsorption process.
The purpose of this investigation is to study lead desorption process in order to demonstrate the ability of exhausted MSG for regeneration and reuse. Micrographs of scanning electron microscopy(SEM), energy- dispersive analysis of X-ray(EDAX) and Fourier transform infrared spectrometry(FTIR) analysis were used to investigate the re-adsorption mechanism of Pb(Ⅱ) by regenerated MSG.
2 Experimental
2.1 Adsorbent preparation
Spent grain was obtained from Yingbo brewery located in Changsha, China. The waste was sun dried and agitated with 1 mol/L NaCl solution (solid to liquid ratio was 100 g/L) at 25 ℃ for 13 h. After then, the solution was filtered and residues were taken out and washed several times with ultrapure water. Modified spent grain named as MSG was dried at 80 ℃ and ground to pass through a 0.355 mm sieve in order to be used as adsorbent.
2.2 Characterization of adsorbent
The surface structures and components of samples were analyzed by JSM-6360LV scanning electronic microscope coupled with Genesis 60S energy dispersive X-ray analyzer. Infrared spectra of regenerated MSG before and after adsorbing Pb(Ⅱ) in solid phase were performed by the KBr method using an AVATAR-360 Fourier transform infrared spectrometer.
2.3 Chemicals
All the chemicals used in this study were of analytic grade. The stock solution of Pb(Ⅱ) was prepared by dissolving Pb(NO3)2 in ultrapure water. A series of Pb(Ⅱ) solutions used in these experiments were made by diluting the stock solutions to the desired concentrations from 20 to 600 mg/L. Before mixing these solutions with the adsorbent, pH values of them were adjusted to 5.5 by adding 0.1 mol/L NaOH or 0.1 mol/L HNO3.
2.4 Adsorption, desorption and re-adsorption experiments
For batch adsorption experiments, 200 mL of Pb(Ⅱ) solution was placed in 250 mL conical flask and set at pH 5.5[19]. An accurately weighed amount (1.2 g) of MSG was added to the solution. The conical flask was then stirred at a constant speed of 120 r/min by a magnetic stirrer in a temperature controlled water bath at 25 ℃. After stirring the flasks for 1 h, MSG was separated by filtration to obtain different loads of Pb(Ⅱ) for desorption. The filtrate was analyzed for the remaining Pb(Ⅱ) concentration by a WFX-120 atomic absorption spectrophotometer.
Batch desorption experiments were conducted using 100 mL conical flask, containing 50 mL of the eluting solution in contact with lead-loaded adsorbent (0.3 g). The suspension was stirred at 120 r/min by a magnetic stirrer in a temperature controlled water bath; 0.5 h later, equilibrium was reached, samples were taken out, and MSG was collected by filtration for reusing it in a new adsorption–desorption cycle. The filtrate was analyzed for the remaining Pb(Ⅱ) concentration by a WFX-120 atomic absorption spectrophotometer. The elution efficiency can be defined as
![]() ×100 (1)
×100 (1)
where η is the elution efficiency; m1 is the mass of metal eluted; m2 is the mass of metal loaded.
The re-adsorption experiments can be performed as the adsorption experiments mentioned above. But, in this case, experiments were started with regenerated MSG (using 0.1 mol/L HCl). After mixing the adsorbent with Pb(Ⅱ) solution (20 mg/L), pH values were adjusted to 5.5 by adding 0.1 mol/L NaOH or 0.1 mol/L HNO3, which was a crucial step impacting the re-adsorption effect. Once the equilibrium was reached, MSG was also collected for reusing it in a new adsorption–desorption cycle.
3 Results and discussion
3.1 Effect of elution agents on lead desorption
Seven different chemical agents of 0.1 mol/L were used for desorbing Pb(Ⅱ) from the lead-loaded MSG. As shown in Fig.1, 0.1 mol/L HCl is the most efficient desorbent since the elution efficiency is higher than 86%, followed in decreasing order by H2SO4, H3PO4, NaOH, ultrapure water and NaCl. In acidic medium, the protons in solution replace the metal ions on the modified spent grain. The apparent poor recovery observed in basic media such as NaOH may be due to the coordinating ligands being deprotonated; hence, bound-metal ions is found difficult to be detached from the modified spent grain. Superiority of ethylenediaminetetraacetic acid (EDTA) in desorbing metal ions from the metal-loaded biomass was also reported in earlier works[20]. However, EDTA cannot be used for metal desorption in a commercial process as it is more costly than HCl; moreover, its disposal may cause serious environment problems. The high elution efficiency, no physical changes or damage on the adsorbent, and the low cost exhibited by HCl indicate that it can be used as an eluant for MSG generation. When HCl is used as a desorption agent, the MSG surface is completely covered by H+ ions while the coordination sphere of chelated Pb(Ⅱ) ions is disrupted. Thereafter, the Pb(Ⅱ) ions can not compete with H+ ions for adsorption sites and subsequently heavy metal ions are released from the solid surface into the solution. At the end of the desorption process, the biomass becomes totally protonated to be ready for the next adsorption cycle.
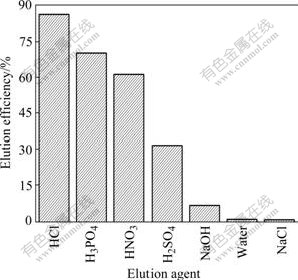
Fig.1 Desorption of lead from lead-loaded MSG by various chemical agents
3.2 Effect of eluant concentration on lead desorption
Elution efficiency using different concentration of HCl as eluant was calculated and results are presented in Fig.2. It can be observed that the maximum elution efficiency is obtained when HCl concentration is 0.1 mol/L. For 0.01 mol/L, the elution efficiency is only 69.22%. Higher value of 86.44% is found for 0.1 mol/L. And then its value decreases a little during the process. Using higher concentration of HCl for lead desorption, compared with 0.1 mol/L, seems to deteriorate the biomass, thereby diminishing its metal sorption. So, 0.1 mol/L is selected as the tested eluant concentration for the rest of the experimental studies.
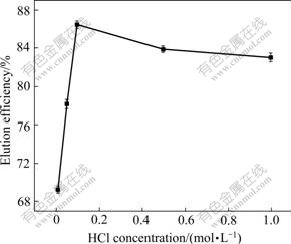
Fig.2 Effect of eluant concentration of HCl on lead desorption
3.3 Effect of desorbing time on lead desorption
Equilibrium time is an important operational parameter for an economical wastewater treatment process. Fig.3 shows the change of lead desorption with desorbing time. Lead desorption increases with increasing contact time until equilibrious desorption is established. Desorption is very rapid and 85.57% of lead desorption occurs during the first 5 min, and then slows down abruptly. The desorption equilibrium is obtained at 15 min. In order to obtain the complete desorption, 30 min is selected as the tested contact time for the rest of the experimental studies.

Fig.3 Effect of desorbing time on lead desorption
3.4 Effect of temperature on different loads of Pb(Ⅱ) desorption
The effect of temperature on the desorption of different loads of Pb(Ⅱ) is presented in Fig.4. On one hand, elution efficiency decreases as temperature increases (15-45 ℃) due to HCl desorption. Decreasing desorption temperature enhances desorption of lead from MSG. On the other hand, the lead elution efficiency also decreases with increasing the initial Pb(Ⅱ) loading at the same temperature. For lower loads of Pb(Ⅱ), Pb(Ⅱ) onto MSG will interact with the eluant binding sites completely and thus facilitate desorption. For higher loads of Pb(Ⅱ), more Pb(Ⅱ) ions without desorption are due to the saturation of eluant binding sites.

Fig.4 Effect of temperature on different with loads of Pb(Ⅱ) desorption
3.5 Successive cycles of Pb(Ⅱ) sorption and desorption
Regeneration of MSG is an important step in order to make the adsorption process more economical. Four successive cycles of sorption and desorption of Pb(Ⅱ) were carried out in the batch system to assess the reusability of MSG for Pb(Ⅱ) sorption (Fig.5). Sorption and desorption of the Pb(Ⅱ) decrease a little after each successive cycle. After four sorption–desorption cycles, the sorption of Pb(Ⅱ) by the MSG only decreases by 1.69% and desorption of Pb(Ⅱ) decreases by 21.93%, respectively. Hence, MSG has better reusability during four successive cycles of sorption and desorption for Pb(Ⅱ). The results show that MSG can be repeatedly used in Pb(Ⅱ) adsorption with slight losses in its initial adsorption efficiency.
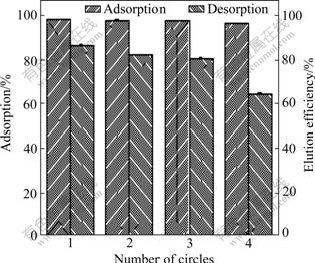
Fig.5 Adsorption–desorption cycles for MSG
3.6 Re-adsorption mechanism
3.6.1 SEM and EDAX analyses
SEM is widely used to study the morphological features and surface characteristics of the adsorbent materials. In this study, the surface morphologies of regenerated MSG before and after adsorbing Pb(Ⅱ) were compared by SEM analysis. An examination of the SEM micrographs (Figs.6(a) and (b)) indicates the presence of many pores on the surface of the adsorbents, and a rough structure on surface with a large surface area. From Fig.6(b), it can be seen that small particles of amorphous precipitate adhere to the MSG surface. This may be due to the presence of lead on the MSG surface, which can be further confirmed from the EDAX analysis. This is thought to be successful adsorption of lead onto regenerated MSG.
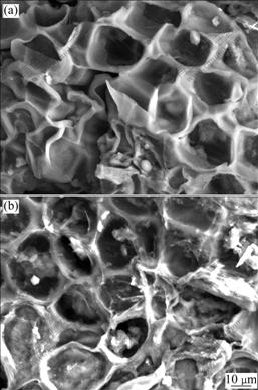
Fig.6 SEM images of regenerated MSG before (a) and after (b) adsorbing Pb(Ⅱ)
EDAX was used to analyze the elemental constitution of solid samples. The EDAX spectrum for regenerated MSG is illustrated in Fig.7(a). C, O, Si, S, and Cl signals can be observed, which are known as the principal elements of MSG. The EDAX spectrum for regenerated MSG adsorbing Pb(Ⅱ) is illustrated in Fig.7(b). C, O, Si, and Pb signals can be observed. The emergence of Pb signal provides direct evidence that lead ions have been adsorbed onto the surface of regenerated MSG.
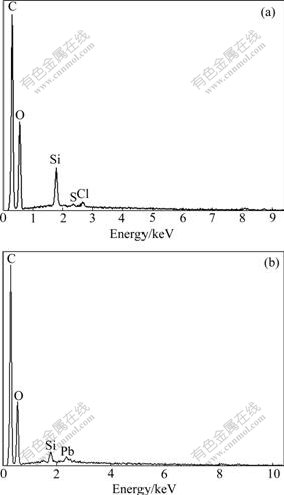
Fig.7 EDAX spectra of regenerated MSG before (a) and after (b) adsorbing Pb(Ⅱ)
3.6.2 FTIR analysis
FTIR spectroscopy has been frequently used to detect vibrational frequency changes in adsorbent. It offers excellent information on the nature of the bonds and allows identification of different functionalities on the adsorbent surface. The assignment of FTIR bands and detailed wavenumber shifts for the regenerated MSG before and after adsorbing Pb(Ⅱ) are summarized in Table 1 while the FTIR spectra obtained for samples are shown in Fig.8. FTIR studies reveal that Pb(Ⅱ) binding to regenerated MSG occurs primarily through carboxyl and hydroxyl groups. Some participation of biomass C—Cl, N—H, C—N and O—H (polysaccharides) in Pb(Ⅱ) binding are also observed.
Table 1 FTIR spectra analysis of regenerated MSG before and after adsorbing Pb(Ⅱ)[21-24]
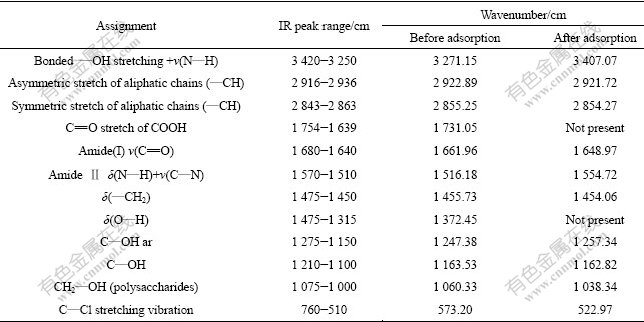
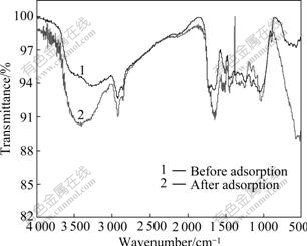
Fig.8 FTIR spectra of regenerated MSG before and after adsorbing Pb(II)
4 Conclusions
1) Lead desorption is very fast, taking less than 15 min with 0.1 mol/L HCl. The lead elution efficiency decreases as temperature increases (15-45 ℃) and also decreases with increasing the loads of Pb(Ⅱ) at the same temperature.
2) MSG can be repeatedly used after four successive cycles of Pb(Ⅱ) sorption and desorption.
3) Pb(Ⅱ) binding to regenerated MSG occurs primarily through carboxyl and hydroxyl groups. C—Cl, N—H, C—N and O—H (polysaccharides) groups also play an important role in Pb(Ⅱ) binding.
References
[1] KUO C Y. Desorption and re-adsorption of carbon nanotubes: Comparisons of sodium hydroxide and microwave irradiation processes [J]. Journal of Hazardous Materials, 2008, 152(3): 949-954.
[2] AJMAL M, HUSSAIN KHAN A, AHMAD S, AHMAD A. Role of sawdust in the removal of copper(Ⅱ) from industrial wastes [J]. Water Research, 1998, 32(10): 3085-3091.
[3] BABEL S, KURNIAWAN T A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review [J]. Journal of Hazardous Materials, 2003, 97(1/3): 219-243.
[4] wan NGAH W S, HANAFIAH M A K M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review [J]. Bioresource Technology, 2008, 99(10): 3935-3948.
[5] CRISAFULLY R, MILHOME M A L, CAVALCANTE R M, SILVEIRA E R, de KEUKELEIRE D, NASCIMENTO R F. Removal of some polycyclic aromatic hydrocarbons from petrochemical wastewater using low-cost adsorbents of natural origin [J]. Bioresource Technology, 2008, 99(10): 4515-4519.
[6] PAVAN F A, MAZZOCATO A C, GUSHIKEM Y. Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent [J]. Bioresource Technology, 2008, 99(8): 3162-3165.
[7] MEMON G Z, BHANGER M I, AKHTAR M, TALPUR F N, MEMON J R. Adsorption of methyl parathion pesticide from water using watermelon peels as a low cost adsorbent [J]. Chemical Engineering Journal, 2008, 138(1/3): 616-621.
[8] MENARD D, PY X, MAZET N. Activated carbon monolith of high thermal conductivity for adsorption processes improvement (Part B): Thermal regeneration [J]. Chemical Engineering and Processing, 2007, 46(6): 565-572.
[9] CHIOU M S, LI H Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads [J]. Chemosphere, 2003, 50(8): 1095-1105.
[10] TANTHAPANICHAKOON W, ARIYADEJWANICH P, JAPTHONG P, NAKAGAWA K, MUKAI S R, TAMON H. Adsorption-desorption characteristics of phenol and reactive dyes from aqueous solution on mesoporous activated carbon prepared from waste tires [J]. Water Research, 2005, 39(7): 1347-1353.
[11] NAKANO Y, HUA L Q, NISHIJIMA W, SHOTO E, OKADA M. Biodegradation of trichloroethylene(TCE) adsorbed on granular activated carbon(GAC) [J]. Water Research, 2000, 34(17): 4139-4142.
[12] SALVADOR F, JIMENEZ C S. A new method for regenerating activated carbon by thermal desorption with liquid water under subcritical conditions [J]. Carbon, 1996, 34(4): 511-516.
[13] QUAN X, LIU X, BO L, CHEN S, ZHAO Y, CUI X. Regeneration of acid orange 7-exhausted granular activated carbons with microwave irradiation [J]. Water Research, 2004, 38(20): 4484-4490.
[14] LIM J L, OKADA M. Regeneration of granular activated carbon using ultrasound [J]. Ultrasonics Sonochemistry, 2005, 12(4): 277-282.
[15] HAMDAOUI O, NAFFRECHOUX E, TIFOUTI L, PETRIER C. Effects of ultrasound on adsorption-desorption of p-chlorophenol on granular activated carbon [J]. Ultrasonics Sonochemistry, 2003, 10(2): 109-114.
[16] SINGH V, TIWARI S, SHARMA A K, SANGHI R. Removal of lead from aqueous solutions using Cassia grandis seed gum-graft-poly (methylmethacrylate) [J]. Journal of Colloid and Interface Science, 2007, 316(2): 224-232.
[17] LI X M, LIAO D X, XU X Q, YANG Q, ZENG G M, ZHENG W, GUO L. Kinetic studies for the biosorption of lead and copper ions by Penicillium simplicissimum immobilized within loofa sponge [J]. Journal of Hazardous Materials, 2008, 159(2/3): 610-615.
[18] SAEED A, IQBAL M, AKHTAR M W. Removal and recovery of lead(Ⅱ) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk) [J]. Journal of Hazardous Materials, 2005, 117(1): 65-73.
[19] LI Q Z, WANG Q W, CHAI L Y, YANG Z H, WANG Y Y. Modification of spent grains to improve Pb2+ adsorption from wastewater [J]. Acta Scientiae Circumstantiae, 2008, 28(6): 1101-1106. (in Chinese)
[20] MARTINS B L, CRUZ C C V, LUNA A S, HENRIQUES C A. Sorption and desorption of Pb2+ ions by dead Sargassum sp. biomass [J]. Biochemical Engineering Journal, 2006, 27(3): 310-314.
[21] MURPHY V, HUGHES H, MCLOUGHLIN P. Cu(Ⅱ) binding by dried biomass of red, green and brown macroalgae [J]. Water Research, 2007, 41(4): 731-740.
[22] GALICHET A, SOCKALINGUM G D, BELARBI A, MANFAIT M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype [J]. FEMS Microbiology Letters, 2001, 197(2): 179-186.
[23] NOLETO G R, MERCE A L R, IACOMINI M, GORIN P A J,OLIVEIRA M B M. Yeast mannan-vanadium(Ⅳ) complexes and their effect on peritoneal macrophages [J]. Carbohydrate Polymers, 2004, 57(2): 113-122.
[24] PRETSCH E, B?HLMANN P, AFFOLTER C. Structure determination of organic compounds tables of spectral data [M]. New York: Springer, 2000: 263-268.
Foundation item: Project(2007BAC25B01) supported by the National Key Project of Science and Technology Supporting Programs of China; Project (50830301) supported by the Key Scientific and Technical Project of Ministry of Education of China
Corresponding author: CHAI Li-yuan; Tel: +86-731-88836921; E-mail: lychai@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60452-5


