Trans. Nonferrous Met. Soc. China 31(2021) 1784-1795
Selective depression of copper-activated sphalerite by polyaspartic acid during chalcopyrite flotation
Qian WEI, Fen JIAO, Liu-yang DONG, Xue-duan LIU, Wen-qing QIN
School of Mineral Processing and Bioengineering, Central South University, Changsha 410083, China
Received 14 July 2020; accepted 30 December 2020
Abstract:
Environmentally friendly flotation reagent, polyaspartic acid (PAPA), was tested as a potential selective depressant in the flotation separation of chalcopyrite and Cu-activated sphalerite. The depression mechanism of PAPA was revealed by contact angle measurements, Zeta potential measurements, Fourier transform infrared spectroscopy (FT-IR) analysis and inductively coupled plasma (ICP) measurement. The micro-flotation tests with single minerals showed that PAPA selectively depressed Cu-activated sphalerite, while chalcopyrite remained floatable. Moreover, a concentrate containing 31.40% Cu with a recovery of 92.43% was obtained in flotation tests of artificially mixed minerals. Results of contact angle measurements, Zeta potential measurements and FT-IR spectrum revealed that PAPA exerted a much stronger adsorption on Cu-activated sphalerite surface than on chalcopyrite surface, preventing the further adsorption of sodium diethyl dithiocarbamate (DDTC) on its surface. ICP measurements indicated that PAPA had an excellent complexing ability with Cu2+ in flotation pulp, weakening the activation of Cu species on sphalerite surface and producing selective depression.
Key words:
chalcopyrite; sphalerite; flotation separation; depressant; polyaspartic acid;
1 Introduction
Chalcopyrite (CuFeS2) and sphalerite (ZnS) are the main copper and zinc minerals found in complex sulfide ores, and they are closely related to economic development. Chalcopyrite always co-exists with sphalerite to form complex Cu-Zn sulfide ore. With the continuous development and utilization of copper-bearing resources, copper ores which are very difficult to treat, due to fine dissemination of chalcopyrite, complex mineralogy, or both, respond very poor to the flotation separation. Conventionally, the Cu-Zn sulfide ore is processed by differential flotation, in which sphalerite is selectively inhibited during chalcopyrite flotation [1-3]. Sphalerite is naturally hydrophilic and has poor floatability, and the adsorption of the short chain collectors on sphalerite surface is weak [4-6]. Because the minerals are finely disseminated and intimately associated with the gangue, they must be initially liberated before flotation separation can be undertaken. During the grinding and flotation process, unavoidable copper ions, generated by the dissolution of oxidized chalcopyrite and secondary copper sulfide ore, always increase the floatability of sphalerite by unwanted activation [7]. As a result, the increase of sphalerite in Cu concentrates declines the copper grade and lowers the zinc recovery. Therefore, to selectively depress the flotation of Cu-activated sphalerite, copper industry needs an effective depressant.
In conventional Cu-Zn flotation separation system, inorganic depressants, including cyanide, zinc sulfate, sulfur oxides, etc, have been widely used for sphalerite depression [8-10]. Cyanide has an excellent depressing effect towards sphalerite; however, cyanide is toxic, and the use of it has raised concerns on environment [11]. Zinc sulfate and sulfur oxides also have some shortcomings, such as poor inhibition performance and large dosage [12]. Organic inhibitors, on the other hand, have advantages of lower dosage, better selectivity and lower pollution. They are environmentally friendly reagents and have been received more and more attentions. FENG et al [13,14] studied the flotation separation of chalcopyrite and sphalerite in the presence of locust bean gum. However, the chalcopyrite recovery was declined to 70% when the dosage of locust bean gum exceeded 400 kg/t, and the solubility of locust bean gum was poor at room temperature. LIU et al [15,16] and QIN et al [17] found that sodium dimethyl dithiocar- bamate (SDD or DMDC) was effective to depress Cu-activated sphalerite/marmatite using butyl xanthate as collector at pH 9.0. However, when DMDC dosage was larger than 1×10-5 mol/L, the recovery of chalcopyrite began to decrease. Obviously, the optimal DMDC concentration range was very narrow, which limited its industrial application. HUANG et al [18] found that the addition of chitosan caused the Cu-coated sphalerite to be inhibited while galena could be floated at pH 4.0. During the flotation process of real ores, depression of pyrite is essential in sulphide mineral flotation. Flotation is carried out in an alkaline medium, but the alkaline pH conditions are not conductive to the depression of chitosan on sphalerite. Hence, it is imperative to develop and choose a natural, non-toxic, bio-degradable Zn depressants for the flotation separation of Cu-activated sphalerite from chalcopyrite.
Polyaspartic acid (PAPA), a kind of polycarboxylic acids, is a new type of green water treatment reagent. Figure 1 shows the chemical structure of PAPA [19]. As presented in Fig. 1, there are a large number of carboxyl groups and hydroxyl groups in PAPA molecular structure, and the carboxyl groups can easily chelate with heavy metal ions. Thus, PAPA shows an excellent scale and corrosion inhibition performance [20,21]. Therefore, PAPA is widely used as dispersant and scale inhibitor in water treatment. In mineral flotation system, DONG et al [22] studied the selective adsorption of sodium polyacrylate (PAAS) on calcite surface in apatite flotation. In the fluorite flotation, polyaspartate (PASP) was used to depress calcite [19,23]. However, little research has been reported on the depressing effect of PAPA in the flotation separation of sulfide mineral flotation, and it is still unclear if PAPA can depress sphalerite during chalcopyrite flotation.
In this study, the flotation separation of sphalerite from chalcopyrite using depressant PAPA and collector DDTC was studied. The flotation behaviors of the two minerals in the absence and presence of reagents were investigated by micro-flotation experiments. The experiment of manually-mixed minerals was carried out to verify the efficiency of actual separating effect of sphalerite from chalcopyrite. Additionally, solution chemical calculation of PAPA solution was employed to investigate the relationship between minerals flotation behavior and solution components. ICP measurements were used to examine the complexing ability of PAPA with metal ions in solution. Moreover, the depression mechanism of PAPA on chalcopyrite and sphalerite was also investigated using contact angle measurements, Zeta potential experiments and FT-IR analysis.
2 Experimental
2.1 Materials
Pure mineral samples of chalcopyrite and sphalerite used in this study were respectively obtained from Hubei and Hunan Province, China. First, raw ores with good crystallinity were handpicked to obtain high purity samples. Then, the selected samples were crushed, dry-ground and dry-sieved to collect the maximum amount of 38-74 μm size fraction for micro-flotation experiments. The samples of <38 μm size fraction were further ground to achieve <2 μm for Zeta potential measurements, FT-IR analysis, chemical multi-element analysis and X-ray diffraction (XRD) analysis.
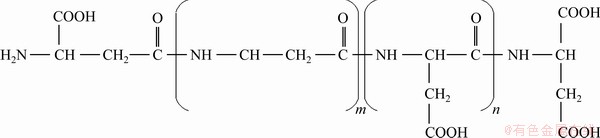
Fig. 1 Molecular structure of PAPA [19]
Table 1 gives the chemical analysis results of the samples. From Table 1, the chalcopyrite and sphalerite samples contained 34.14% Cu and 65.42% Zn, respectively, indicating that the chalcopyrite and sphalerite were 98.78% and 97.50% pure, respectively. Figure 2 shows the XRD patterns of the analyzed studied mineral samples. As shown in Fig. 2, both minerals showed high purity.
The polyaspartic acid (PAPA, analytical grade) was employed as the depressant and was purchased from Shandong Taihe Water Treatment Technologies Co., Ltd., China. The collectors of sodium diethyl dithiocarbamate (DDTC, industry grade) and frother terpineol oil (industry grade) were purchased from Mingzhu Flotation Reagents Factory in Hunan Province, China. Copper sulfate (CuSO4, activator) and zinc sulfate (ZnSO4) were purchased from Tianjin Yongda Chemical Reagent Co., Ltd., China. Stock solutions of hydrochloric acid (HCl) and sodium hydroxide (NaOH) were prepared for pH adjustment. All the chemical reagents utilized in this study, except for DDTC and terpenic oil, were of analytical grade. Deionized (DI) water with the resistivity over 18.0 MΩ·cm was used for all experiments.
2.2 Micro-flotation experiments
All the micro-flotation experiments were performed using laboratory XFG series flotation machine (Jilin Exploration Equipment, Changchun, China) with a flotation cell (effective volume 40 mL). The spindle speed of the flotation machine was fixed at 1690 r/min. The procedure of micro-flotation was as follows. First, 2.0 g (38-74 μm) of the sample was pre-cleaned for 5 min by ultrasonic treatment and then mixed with 35 mL DI water in flotation cell. After stirring for 1 min and adjusting pH value for 2 min, the activator CuSO4, the depressant PAPA, the collector DDTC and the frother terpineol oil were added to the mineral suspension in sequence, and the conditioning time of flotation reagents was 2 min, 2 min, 2 min and 1 min, respectively, as shown in Fig. 3. Finally, the concentrates and tailings were separately collected, dried and then weighted, and the recovery was calculated using Eq. (1). Each experiment was repeated three times and averaged.
Table 1 Chemical analysis results of two mineral samples
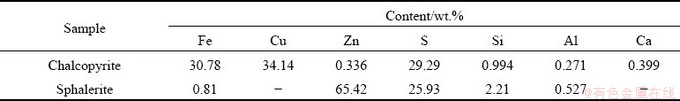
Fig. 2 XRD patterns of chalcopyrite (a) and sphalerite (b)
 (1)
(1)
where ε represents flotation recovery (%); m1 and m2 represent masses (g) of concentrates and tailings, respectively.
For the flotation separation of artificial mixed minerals, 1.0 g chalcopyrite and 1.0 g sphalerite were mixed into manually mixed minerals. After the artificially mixed minerals were stirred for 1 min, then the desired concentration of flotation reagents was added, and the addition sequence and reaction time were consistent with those of single mineral flotation experiments. The concentrates and tailings were separately weighed and the yield (wt.%) was calculated. Flotation recovery was calculated according to the yields of the two products and the metal grade assessed by chemical analysis.
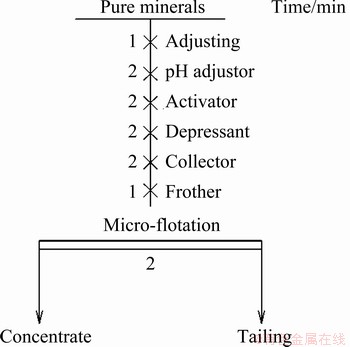
Fig. 3 Flowsheet of micro-flotation experiments
2.3 Contact angle measurements
Contact angle measurements were employed to evaluate the wettability of mineral surfaces before and after interaction with flotation reagents. First, a lump of pure mineral with high purity and good crystallization was selected, cut and ground to obtain a suitable size (approximately 2.0 cm × 2.0 cm × 1.0 cm). In order to minimize the effect of surface roughness on contact angle measurement, after being cut and ground, the prepared sample was further sequentially polished by Wuxi brand sandpaper, from coarse to fine. Sessile drop method was utilized to determine the contact angle of DI water on the prepared sample surface, and JY-82C automatic video contact angle meter (Dingsheng Tester) was used in this work. The prepared sample was mixed with 35 mL DI water, agitated and reacted with a desired concentration of flotation reagents in a beaker, then removed by a tweezer and repeatedly rinsed three times with DI water, dried by nitrogen, and put on the stage of the instrument. During each measurement, a suitable and stable DI water drop was deposited on the sample surface, and the contact angle was measured by capturing the microscopic image between the drop and the prepared mineral surface.
2.4 Zeta potential measurements
Zeta potential analyzer (Malvern Instruments, UK) was utilized to analyze the Zeta potential of mineral samples in the absence and present of the desired amount of flotation reagents. For each test, 20 mg (<2 μm) sample was added to a beaker with 35 mL KCl supporting electrolyte solution (1×10-3 mol/L), and agitated for 1 min. Following the pH adjustment with NaOH or HCl with a stirring time of 2 min, the flotation reagent(s) was/were added, and the addition sequence, reaction time and the concentration of the reagents were consistent with those of the micro-flotation tests. The slurry was stood for about 10 min to settle large particles. Finally, the suspension containing fine mineral particles was pipetted out by a transfer pipette for Zeta potential measurements.
2.5 FT-IR analysis
Infrared spectral (IR) analyses of mineral samples in absence and presence flotation reagents were recorded by IRAffinity-1 spectrometer (Shimadzu Corporation, Kyoto, Japan). The IR spectra were detected by using KBr tableting methods in transmission mode, and the wave number was in the range from 4000 to 400 cm-1. 1.0 g (<2 μm) of the sample and 35 mL DI water were mixed in a beaker, and agitated for 2 min by a magnetic stirrer to thoroughly disperse the mineral particles in DI water. The preparation methods of the mineral samples in the presence of flotation reagents were consistent with those of micro- flotation experiments. After the reaction was completed, the suspension was filtered. Finally, the treated samples were washed three times with DI water and dried in a vacuum desiccator prior to IR spectral measurements.
2.6 ICP measurements
ICP measurements were employed to investigate the chelating ability of PAPA with metal ions, and the concentration of copper ions and zinc ions was determined by the residual concentration method. 0.2 g of CuSO4·5H2O (or ZnSO4·7H2O) was mixed with 20 mL DI water in a beaker to prepare simulated copper ion solution (or zinc ion solution). Six parts of copper ion solution (or zinc ion solution) at the same concentration were prepared according to the same configuration method. Subsequently, PAPA solutions at six given concentrations (5, 10, 20, 40, 60 and 80 mg/mL) were respectively added to each of the above six beakers. Afterwards, the solutions were evenly agitated and stood for about 20 min. Next, the suspensions were filtered, and 2 mL of the filtrate was pipetted out and diluted to 5000 mL in a beaker. Then, the diluted solution was measured for Cu2+ (Zn2+) concentration using inductive coupled plasma (ICP; Thermo Scientific; iCAP 6500). The removal rates of Cu2+ (Zn2+) in solution were calculated as
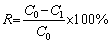 (2)
(2)
where R is removal rate of metal ions (%); C0 and C1 are concentrations (mg/L) of metal ion concentration before and after interaction with PAPA, respectively.
3 Results and discussion
3.1 Microflotation with single minerals
Figure 4(a) shows the flotation recovery of chalcopyrite and sphalerite as a function of pH. In this study, CuSO4 was added to simulate the unwanted activation. In the absence of CuSO4, the recovery of sphalerite slightly declined with an increase in the pH range from 3 to 11.5. The highest recovery of sphalerite in the presence of DDTC alone was only 30.39% at pH 5. This indicated that sphalerite had inferior floatability on its own. In the presence of 3.5×10-5 mol/L CuSO4, DDTC had a good collecting ability for both minerals. The recovery of chalcopyrite was over 95% within the tested pH range, while sphalerite recovery maintained almost constant at around 94% with increasing pH and subsequently decreased from 92.59% to 59.92% with further increase in pH from 9.0 to 11.5. Under alkaline pH conditions, sphalerite recovery gradually decreased when DDTC was used as collector. The main reasons were possibly listed as follows. On one hand, when pH was below 9.0, the ions on mineral surface may be cationic, and the mineral surface was positively charged. Under high alkaline pH conditions, the anions may be positioned on the sphalerite surface, and it was difficult for anionic collector DDTC to adsorb on its surface [17]. On the other hand, because of the hydrolysis of Zn released from sphalerite, various hydroxy complexes were formed, under the studied pH range (9.0-11.5), and the main zinc species of Zn in the aqueous solutions were Zn(OH)2(aq), 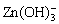 and
and 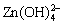 . The hydroxides wrapped on the sphalerite surface, which increased the hydrophilicity of the surface and reduced the floatability [24]. Those results demonstrated that the flotation separation of chalcopyrite and Cu-activated sphalerite was impossible without the addition of a depressant. After the addition of PAPA prior to DDTC, Cu-activated sphalerite dramatically decreased from 93.22% to the minimum of 29.59% with the increase of pH from 3 to 9 and subsequently slightly increased to 49.78% with further increase in pH to 12.0. In contrast, PAPA had little effect on the floatability of chalcopyrite. The large floatability difference between chalcopyrite and Cu-activated sphalerite in the presence of PAPA presented the possibility of flotation separation of the two minerals. Figure 4(b) shows the effect of PAPA concentration on the flotation recovery of chalcopyrite and Cu-activated sphalerite. From Fig. 4(b), in the absence of the depressant PAPA, the recovery of both minerals were above 90% at pH 9.0, and the flotation separation of the two minerals was difficult in this case. Sphalerite recovery was sharply depressed by PAPA, with its recovery significantly decreasing from 92.91% to 19.91% with 16 mg/L of PAPA. However, the floatability of chalcopyrite was not influenced, remaining at around 95% in the whole tested PAPA dosage. Under these conditions, the flotation separation of chalcopyrite from Cu-activated sphalerite could be achieved by flotation.
. The hydroxides wrapped on the sphalerite surface, which increased the hydrophilicity of the surface and reduced the floatability [24]. Those results demonstrated that the flotation separation of chalcopyrite and Cu-activated sphalerite was impossible without the addition of a depressant. After the addition of PAPA prior to DDTC, Cu-activated sphalerite dramatically decreased from 93.22% to the minimum of 29.59% with the increase of pH from 3 to 9 and subsequently slightly increased to 49.78% with further increase in pH to 12.0. In contrast, PAPA had little effect on the floatability of chalcopyrite. The large floatability difference between chalcopyrite and Cu-activated sphalerite in the presence of PAPA presented the possibility of flotation separation of the two minerals. Figure 4(b) shows the effect of PAPA concentration on the flotation recovery of chalcopyrite and Cu-activated sphalerite. From Fig. 4(b), in the absence of the depressant PAPA, the recovery of both minerals were above 90% at pH 9.0, and the flotation separation of the two minerals was difficult in this case. Sphalerite recovery was sharply depressed by PAPA, with its recovery significantly decreasing from 92.91% to 19.91% with 16 mg/L of PAPA. However, the floatability of chalcopyrite was not influenced, remaining at around 95% in the whole tested PAPA dosage. Under these conditions, the flotation separation of chalcopyrite from Cu-activated sphalerite could be achieved by flotation.
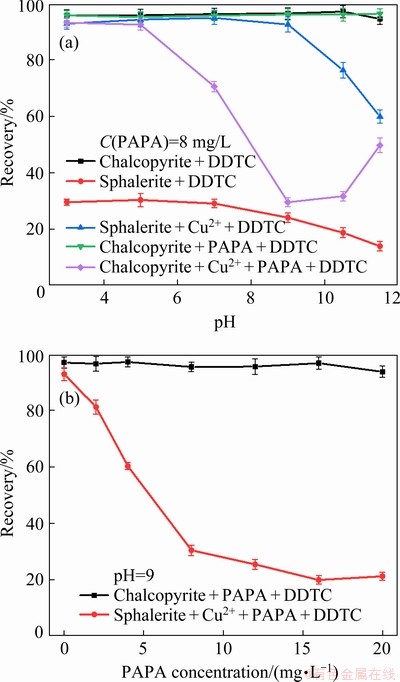
Fig. 4 Flotation recovery of chalcopyrite and sphalerite as function of pH (a) and PAPA concentration (b) (C(CuSO4)=3.5×10-5 mol/L, C(DDTC)=3×10-5 mol/L, and C(MIBC)=10 mg/L)
3.2 Microflotation with mixed minerals
To further verify the actual depressing effect of PAPA on sphalerite during chalcopyrite flotation, the flotation separation of artificially mixed binary minerals (mass ratio of 1:1) was carried out, and the results are given in Table 2. According to Table 2, when the depressant PAPA was not added, the Cu grade and Zn grade in Cu concentrate were 15.51% and 29.55%, respectively, which were similar with the Cu grade and Zn grade of 15.13% and 33.21% of the feed sample. Moreover, the Cu recovery and Zn recovery in Cu concentrate were 92.60% and 80.37%, respectively. This indicated that the flotation separation of the two minerals was insignificant. However, when 16 mg/L PAPA was added, the Cu grade in Cu concentrate significantly increased from 15.51% to 31.40%, with the Cu recovery of 92.43%. Obviously, the copper recovery basically unchanged (maintained at about 92%) in the presence of depressant PAPA. Therefore, by adding PAPA as depressant, the copper grade could be greatly increased without affecting the Cu recovery. This indicated that PAPA had an excellent depressing effect on sphalerite during chalcopyrite flotation, which was in good agreement with the results of micro-flotation results (single minerals).
3.3 Contact angle
The wettability of the mineral surfaces characterized by contact angle is a direct indicative of the hydrophilic and hydrophobic properties of mineral surfaces. Figure 5 shows the contact angles of chalcopyrite and sphalerite before and after treatment with different flotation reagents. The PAPA concentration was 16 mg/L, and the pH was fixed at 9.0. Figure 5 showed that the contact angle of natural chalcopyrite was 66.44°. After the treatment with PAPA, the contact angle of treated chalcopyrite surface was 65.98°, which was not much changed compared with that of the natural chalcopyrite (66.44°). This indicated that the hydrophobicity and floatability of the chalcopyrite were still good using PAPA as depressant. In the presence of depressant PAPA and collector DDTC, the contact angle was 69.62°. This slight increase in the contact angle implied that the addition of PAPA had little effect on the hydrophobicity of the chalcopyrite surface in the present of collector DDTC. Therefore, under these conditions, chalcopyrite still had a good floatability.
Table 2 Results of flotation separation of artificially mixed minerals
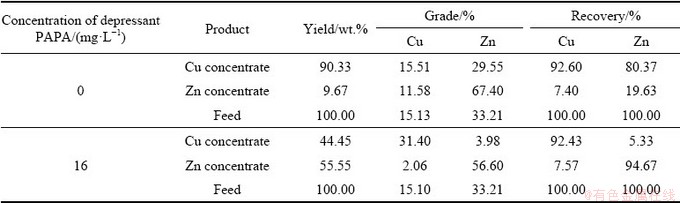
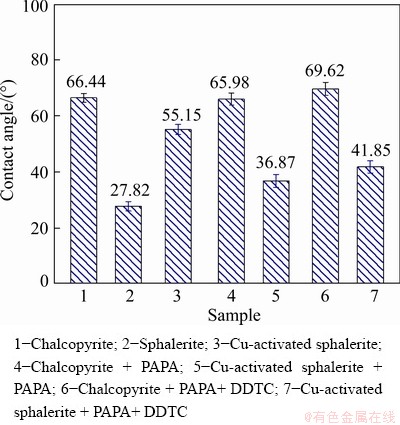
Fig. 5 Contact angles of chalcopyrite and sphalerite in the absence and presence of different flotation reagents (C(CuSO4)=3.5×10-5 mol/L, C(DDTC)=3×10-5 mol/L)
The contact angle of natural sphalerite was 27.82°. After the addition of activator CuSO4, the contact angle of sphalerite surface significantly increased from 27.82° to 55.15°, indicating that the hydrophobicity and floatability of sphalerite were largely enhanced by adding activator CuSO4. In the presence of depressant PAPA, the measured contact angle of Cu-activated sphalerite declined from 55.15° to 36.87°, implying that the hydrophobicity and floatability of the Cu-activated sphalerite surface were obviously reduced after the treatment with PAPA. After the addition of depressant PAPA and then collector DDTC, the measured contact angle of Cu-activated sphalerite slightly increased to 41.85°. Under this condition, the contact angle of Cu-activated sphalerite (41.85°) and the contact angle of Cu-activated sphalerite treated by PAPA (36.87°) were not much different. These results indicated that further adsorption of DDTC on Cu-activated sphalerite surface was prevented by pre-adsorption of PAPA, but the adsorption of DDTC on chalcopyrite pre-treated by PAPA was almost not influenced. This was in good agreement with the micro-flotation results, in which the depressant PAPA selectively decreased the floatability of Cu-activated sphalerite.
3.4 Zeta potential
The adsorption of flotation reagents on mineral surfaces can change the electro-kinetic properties of minerals, thus influencing their floatability [25-27]. Zeta potentials of chalcopyrite and sphalerite as a function of pH in the absence and presence of flotation reagents are shown in Fig. 6. According Fig. 6, the isoelectric point (IEP) of chalcopyrite was at around pH 2.5, and Cu-activated sphalerite was negatively charged in entire experimental pH range, which was agreed well with previous literature reports [28,29]. From Fig. 6(b), in the presence of activator CuSO4 alone, the Zeta potentials of sphalerite steadily decreased from -33.89 to -38.75 mV with the increase of pH from 2 to 6 and subsequently increased to the maximum of -31.29 mV with further increase in pH to 10, and then declined with pH increasing after reaching a maximum. The increase of the Zeta potential of sphalerite at pH 6-10 was attributed to the adsorption of positive copper hydrolyzed products (Cu(OH)+ and Cu2(OH)22+) on sphalerite surface,whereas the decline of Zeta potential at pH>10 was resulted from the adsorption and precipitation of negative copper hydrolyzed products (Cu(OH)3-) on its surface [30].
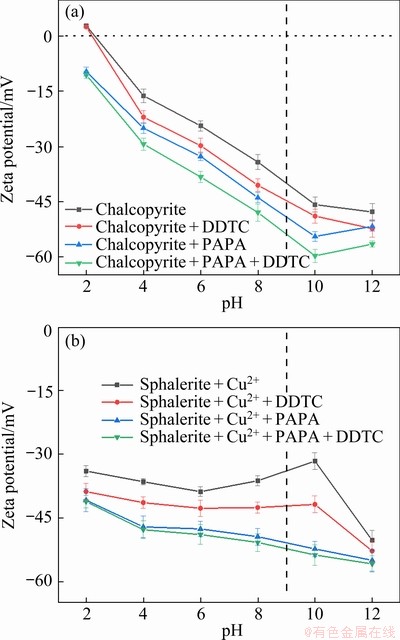
Fig. 6 Zeta potential of chalcopyrite (a) and sphalerite (b) as function of pH before and after treatment with flotation reagents
According to Fig. 6, after being treated with DDTC alone, the Zeta potential of chalcopyrite and Cu-activated sphalerite decreased by approximately 6 and 7 mV due to the adsorption of negatively charged DDTC species on the two mineral surfaces. After the addition with PAPA alone, the Zeta potentials of both minerals moved to the negative direction, indicating that the negatively charged species in PAPA solution were adsorbed on the two mineral surfaces [19]. For Cu-activated sphalerite, the addition of PAPA caused the decrease of 8.85-21.08 mV at pH of 4-10 (Fig. 6(b)) compared to a decrease of 7.58-8.16 mV for chalcopyrite (Fig. 6(a)), implying the much stronger affinity of PAPA towards Cu-activated sphalerite. And the subsequent addition of DDTC further decreased the Zeta potentials of PAPA treated chalcopyrite by around 6 mV while that of PAPA adsorbed Cu-activated sphalerite decreased by only about 1 mV. The results indicated that the adsorption of PAPA was much stronger on the surface of Cu-activated sphalerite than on the surface of chalcopyrite. Moreover, the PAPA adsorbed on Cu-activated sphalerite inhibited the further adsorption of the collector DDTC and thus caused the pyrite depression. However, due to the weak adsorption of PAPA, chalcopyrite surface adsorbed a great amount of the collector DDTC, which maintained its floatability even in the present of the depressant PAPA. These results were consistent with those of the micro-flotation experiments and contact angle measurements.
3.5 FT-IR analysis result
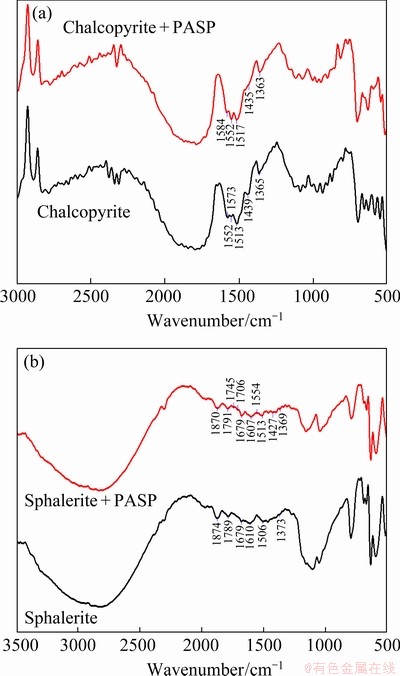
Fig. 7 IR spectra of chalcopyrite (a) and sphalerite (b) before and after treatment with PAPA
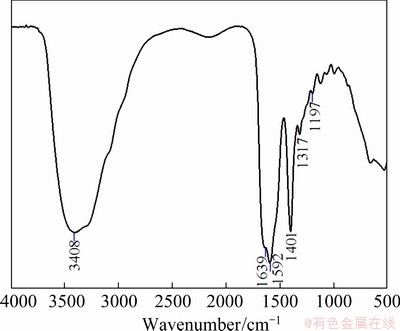
Fig. 8 FT-IR spectrum of PAPA
In order to investigate the interaction mechanism of depressant PAPA on mineral surfaces, IR spectra of both minerals before and after interaction with PAPA at pH 9.0 are shown in Fig. 7. The IR spectrum of PAPA is shown in Fig. 8. As shown in Fig. 8, for PAPA, the adsorption peaks at 1401 and 1592 cm-1 were attributed to the symmetric and antisymmetric stretching vibrations by C=O vibration couplings of —COO- of PAPA, respectively. The adsorption band at 1639 cm-1 was assigned to the characteristic peak of amide group (C=O vibration adsorption peak). And adsorption band at 3408 cm-1 was the characteristic adsorption peak of VNH [31,32]. Combined with the relative molecular mass of PAPA, it could be inferred that PAPA was carboxylate polymer with small molecule [19]. These results are in good agreement with previous studies.
As depicted in Fig. 7(a), with the addition of PAPA, compared with the IR spectrum of untreated chalcopyrite, no new characteristic peak appeared, and the adsorption band shifted from 1573 to 1584 cm-1, which was caused by the adsorption peak of —COO- group in PAPA at 1592 cm-1. The results demonstrated the adsorption of PAPA on chalcopyrite surface. In contrast, from Fig. 7(b), after the interaction between sphalerite and PAPA, main characteristic peaks of PAPA corresponding to C=O vibration adsorption peak of amide group in PAPA (1706 and 1745 cm-1) and C=O vibration couplings of —COO- group in PAPA (1427 and 1554 cm-1) appeared.
To summarize, after the addition of PAPA, no new characteristic adsorption peaks were observed on chalcopyrite surface. But several new and pronounced characteristic adsorption peaks were observed on sphalerite surface. These results closely followed the results of Zeta potential measurements and indicated that the adsorption of PAPA on sphalerite surface was much stronger than that on chalcopyrite surface.
3.6 ICP result
According to relevant literatures [33,34], the chemical structure of PAPA contains several carboxyl groups (as shown in Fig. 1), which renders its hydrophilic characteristics, the ability of complexation with heavy metal ions and adsorption on mineral surfaces. To study the chelating ability of PAPA with metal ions, the removal rate of metal ions as a function of PAPA concentration was measured, and the results are shown in Fig. 9. As indicated, the removal rate of Cu2+ was much greater than that of Zn2+ under the same PAPA concentration. After the addition of PAPA, removal rate of Cu2+ dramatically increased from 25.67% to the maximum of 71.53% with the increase of PAPA dosage from 5 to 20 mg/L and subsequently decreased to the minimum of 1.02% at the PAPA dosage of 80 mg/L. And the removal rate of Zn2+ was much lower than that of Cu2+ and was less than 25%, indicating that the complexing ability of PAPA with Zn2+ was relatively weak. The results indicated that PAPA could weaken the unwanted activation of Cu2+ on sphalerite surface and thus induced selective depression.
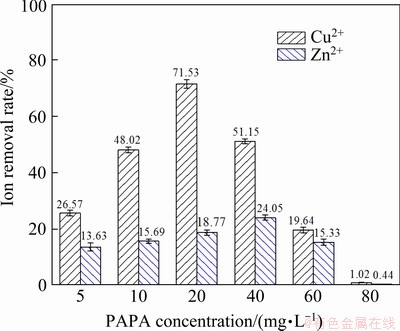
Fig. 9 Removal rates of copper and zinc ions as function of PAPA concentration
3.7 Solution chemical calculation of components of PAPA solution
Table 3 Reactions and corresponding reaction constants
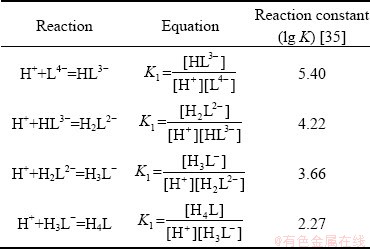
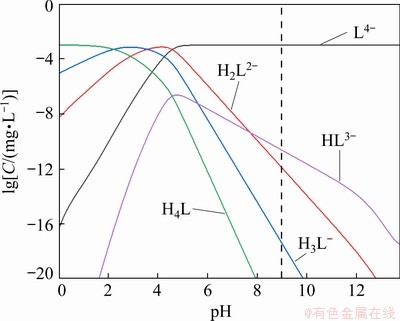
Fig. 10 Species distribution diagram of PAPA solution
In order to further clarify the adsorption mechanism of PAPA on mineral surfaces, solution chemical calculation of the dissolved species of PAPA was carried out. According to previous literatures [18,35], PAPA can be treated as a molecular model with four acid structure units (COCHCH2COOHNH), and thus, the total relative molecular mass is 460. Therefore, only the dissociation of H is considered in carboxyl groups, and the model can be simplified as H4L [34]. The reaction formulas and the corresponding reaction constants are given in Table 3. And the species distribution diagram of PAPA is shown in Fig. 10. As shown in Fig. 10, the dominant component in PAPA solution was L4- at pH 9.0. And the possible chelation reactions between L4- and M2+ (Cu2+/Zn2+) are given in Eqs. (3) and (4). Therefore, at tested pH value of 9.0, anionic species L4- of PAPA could complex with unwanted Cu2+ in flotation pulp to form CuL2- and Cu2L, weakening the activation of Cu2+ on sphalerite surface and producing selective depression. On the other hand, in our previous study [20], a stronger interaction occurred between the combined depressant polyaspartic acid/zinc sulfate (PZ) and Cu-activated marmatite surface through the copper atoms. As well known, the inhibitory effect of zinc sulfate on sphalerite surface was mainly due to the precipitation of hydrophilic zinc hydroxide on its surface in alkaline pH range [20]. Therefore, the interaction between negatively charged L4- of PAPA and Cu atoms on Cu-activated sphalerite surface was probably considered to promote the adsorption of PAPA.
L4-+M2+=ML2- (3)
L4-+2M2+=M2L (4)
4 Conclusions
(1) Within the pH range of 9.0-10.5, the flotation separation of chalcopyrite from Cu-activated sphalerite could be achieved in the presence of PAPA as depressant (16 mg/L) and DDTC as collector (3×10-5 mol/L). From artificially mixed mineral experiments, after the addition of 16 mg/L PAPA, the Cu grade in Cu concentrate was significantly improved from 15.51% to 31.40% compared to that obtained in the absence of PAPA at pH 9.0, while the Cu recovery was not changed much and maintained at around 92%, indicating that PAPA was an effective and selective depressant for Cu-activated sphalerite during chalcopyrite flotation.
(2) FT-IR analysis and Zeta potential measurements indicated the much stronger affinity of PAPA towards Cu-activated sphalerite surface, resulting in a more hydrophilic surface. Moreover, the addition of PAPA could largely prevent the further adsorption of DDTC on the Cu-activated sphalerite surface but exert little effect on the DDTC adsorption on the chalcopyrite surface, as presented by the results of the contact angle measurements.
(3) ICP analysis indicated that PAPA could complex with copper ions in flotation slurry, weakening the accidental activation of copper ions on sphalerite surface, and thus, resulted in the selective inhibition for Cu-activated sphalerite.
Acknowledgments
The authors are grateful for the financial supports from the National Natural Science Foundation of China (Nos. 51974364, 51904339), and Hunan Province for Clean and Efficient Utilization of Strategic Calcium-containing Mineral, China (No. 2018TP1002).
References
[1] QIN Wen-qing, JIAO Fen, SUN Wei, HE Ming-fei, HUANG Hong-jun. Selective flotation of chalcopyrite and marmatite by MBT and electrochemical analysis [J]. Industrial & Engineering Chemistry Research, 2012, 51: 11538-11546.
[2] LOPEZ R, JORDO H, HARTMANN R, MML A, CARVALHO M T. Study of butyl-amine nanocrystal cellulose in the flotation of complex sulphide ores [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 579: 123655.
[3] CUI Yan-fang, JIAO Fen, QIN Wen-qing, DONG Liu-yang, WANG Xu. Synergistic depression mechanism of zinc sulfate and sodium dimethyl dithiocarbamate on sphalerite in Pb-Zn flotation system [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 2547-2555.
[4] WANG Han, WEN Shu-ming, HAN Guang, FENG Qi-cheng. Effect of copper ions on surface properties of ZnSO4- depressed sphalerite and its response to flotation [J]. Separation and Purification Technology, 2019, 228: 115756.
[5] JIAO Fen, WU Jia-jia, QIN Wen-qing, WANG Xing-jie, LIU Run-zeng. Interactions of tert dodecyl mercaptan with sphalerite and effects on its flotation behavior [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 506: 104-113.
[6] BAGHERI B, MEHRABANI J V, FARROKHPAY S. Recovery of sphalerite from a high zinc grade tailing [J]. Journal of Hazardous Material, 2020, 381: 120946.
[7] LIU Run-qing, SUN Wei, HU Yue-hua, WANG Dian-zuo. New collectors for the flotation of unactivated marmatite [J]. Minerals Engineering, 2010, 23: 99-103.
[8] CAO Ming-li, LIU Qi. Reexamining the functions of zinc sulfate as a selective depressant in differential sulfide flotation-The role of coagulation [J]. Journal of Colloid Interface Science, 2006, 301: 523-31.
[9] PULIDO G I D, SALAS A U, GOMEZ R E. Comparison of the depressant action of sulfite and metabisulfite for Cu-activated sphalerite [J]. International Journal of Mineral Processing 2011, 101: 71-74.
[10] ZHAO Cui-hua, HUANG De-wei, CHEN Jian-hua, LI Yu-qiong, CHEN Ye, LI Wei-zhou. The interaction of cyanide with pyrite, marcasite and pyrrhotite [J]. Minerals Engineering, 2016, 95: 131-137.
[11] LIU Run-zeng, QIN Wen-qing, JIAO Fen, WANG Xing-jie, YANG Yong-jun, LAI Chun-hua. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 265-271.
[12] RASHCHI F, FINCH J A, SUI C. Action of DETA, dextrin and carbonate on lead-contaminated sphalerite [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2004, 245: 21-27.
[13] FENG Bo, ZHONG Chun-hui, ZHANG Liang-zhu, GUO Yu-tao, WANG Tao, HUANG Zhi-qiang. Effect of surface oxidation on the depression of sphalerite by locust bean gum [J]. Minerals Engineering, 2020, 146: 106142.
[14] FENG Bo, GUO Yu-tao, ZHANG Wen-pu, PENG Jin-xiu, WANG Hui-hui, HUANG Zhi-qiang, ZHOU Xiao-tong. Flotation separation behavior of chalcopyrite and sphalerite in the presence of locust bean gum [J]. Minerals Engineering, 2019, 143: 105940.
[15] LIU Jian, WANG Yu, LUO De-qiang, ZENG Yong, WEN Shu-ming, CHEN Lu-zheng. DFT study of SDD and BX adsorption on sphalerite (110) surface in the absence and presence of water molecules [J]. Applied Surface Science, 2018, 450: 502-508.
[16] LIU Jian, WANG Yu, LUO De-qiang, ZENG Yong. Use of ZnSO4 and SDD mixture as sphalerite depressant in copper flotation [J]. Minerals Engineering, 2018, 121: 31-38.
[17] QIN Wen-qing, JIAO Fen, SUN Wei, WANG Xing-jie, LIU Bei, WANG Jun, ZENG Ke, WEI Qian, LIU Kai. Effects of sodium salt of N,N-dimethyldi-thiocarbamate on floatability of chalcopyrite, sphalerite, marmatite and its adsorption properties [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 421: 181-192.
[18] HUANG Peng, CAO Ming-li, LIU Qi. Selective depression of sphalerite by chitosan in differential PbZn flotation [J]. International Journal of Mineral Processing, 2013, 122: 29-35.
[19] ZHU Hai-ling, QIN Wen-qing, CHEN chen, CAI Li-yuan, JIAO Fen, JIA Wen-hao. Flotation separation of fluorite from calcite using polyaspartate as depressant [J]. Minerals Engineering, 2018, 120: 80-86.
[20] WEI Qian, JIAO Fen, QIN Wen-qing, DONG Liu-yang, FENG Li-qing. Use of PASP and ZnSO4 mixture as depressant in the flotation separation of chalcopyrite from Cu-activated marmatite [J]. Physicochemical Problems of Mineral Processing, 2019, 55: 1192-1208.
[21] CHEN Jian-xin, XU li-hua, HAN Jian, SU Min, WU Qing. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity [J]. Desalination 2015, 358: 42-48.
[22] DONG Liu-yang, WEI Qian, QIN Wen-qing, JIAO Fen. Selective adsorption of sodium polyacrylate on calcite surface: Implications for flotation separation of apatite from calcite [J]. Separation and Purification Technology, 2020, 241: 116415.
[23] CHEN Chen, HU Yue-hua, ZHU Hai-ling, SUN Wei, QIN Wen-qing, LIU Run-qing, GAO Zhi-yong. Inhibition performance and adsorption of polycarboxylic acids in calcite flotation [J]. Minerals Engineering, 2019, 133: 60-68.
[24] KHOSO S A, HU Yue-hua, LU Fei, GAO Ya, LIU Run-qing, SUN Wei. Xanthate interaction and flotation separation of H2O2-treated chalcopyrite and pyrite [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 2604-2614.
[25] HAN Guang, WEN Shu-ming, WANG Han, FENG Qi-cheng. Selective adsorption mechanism of salicylic acid on pyrite surfaces and its application in flotation separation of chalcopyrite from pyrite [J]. Separation and Purification Technology, 2020, 240: 116650.
[26] ZHAO Wen-juan, LIU Dian-wen, FENG Qi-cheng. Enhancement of salicylhydroxamic acid adsorption by Pb(II) modified hemimorphite surfaces and its effect on floatability [J]. Minerals Engineering, 2020, 152: 106373.
[27] ZHU Hai-ling, QIN Wen-qing, CHEN Chen, CHAI Li-yuan, LI Lai-shun, LIU San-jun, Zhang Ting. Selective flotation of smithsonite, quartz and calcite using alkyl diamine ether as collector [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 163-168.
[28] WANG Dao-wei, JIAO Fen, QIN Wen-qing, WANG Xing-jie. Effect of surface oxidation on the flotation separation of chalcopyrite and galena using sodium humate as depressant [J]. Separation Science and Technology 2017, 53: 961-972.
[29] LIU Run-qing, HU Yue-hua, WANG Dao-wei. Surface chemical study of the selective separation of chalcopyrite and marmatite [J]. Mining Science and Technology (China), 2010, 20: 542-545.
[30] LI Jian-min, SONG Kai-wei, LIU Dian-wen, ZHANG Xiao-lin, LAN Zhuo-yue, SUN Yun-li, WEN Shu-ming. Hydrolyzation and adsorption behaviors of SPH and SCT used as combined depressants in the selective flotation of galena from sphalerite [J]. Journal of Molecular Liquids, 2017, 231: 485-490.
[31] KOODYSKA D, HUBICKI Z, GCA M. Application of a new-generation complexing agent in removal of heavy metal ions from aqueous solutions [J]. Industrial & Engineering Chemistry Research, 2008, 47: 3192-3199.
[32] KOLODYNSKA D. Application of strongly basic anion exchangers for removal of heavy metal ions in the presence of green chelating agent [J]. Chemical Engineering Journal, 2011, 168: 994-1007.
[33] KOLODYNSKA D. The effect of the novel complexing agent in removal of heavy metal ions from waters and waste waters [J]. Chemical Engineering Journal, 2010, 165: 835-845.
[34] KOLODYNSKA D. Chitosan as an effective low-cost sorbent of heavy metal complexes with the polyaspartic acid [J]. Chemical Engineering Journal, 2011, 173: 520-529.
[35] WU You-ting, GRANT C. Effect of chelation chemistry of sodium polyaspartate on the dissolution of calcite [J]. Langmuir, 2002, 18: 6813-6820.
聚天冬氨酸在黄铜矿浮选体系中对铜离子活化后的闪锌矿的选择性抑制作用
魏 茜,焦 芬,董留洋,刘学端,覃文庆
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:采用环保型的浮选药剂聚天冬氨酸(PAPA)作抑制剂,进行黄铜矿和铜活化后闪锌矿的浮选分离。通过接触角测试、动电位测试、红外光谱分析和电感耦合等离子体发射光谱测试,研究PAPA对闪锌矿的选择性抑制机理。单矿物浮选试验结果表明,PAPA能选择性抑制铜活化后的闪锌矿,但黄铜矿仍能保持好的可浮性。人工混合矿浮选试验获得铜精矿中铜品位为31.40%,铜回收率为92.43%。接触角测试、动电位试验和红外光谱分析表明,PAPA在铜活化后的闪锌矿表面吸附更强;另外,PAPA的存在能阻止铜活化后的闪锌矿表面捕收剂二乙基二硫代氨基甲酸钠(DDTC)的进一步吸附。电感耦合等离子体发射光谱测试结果表明,PAPA对铜离子有较强的络合能力,能减弱闪锌矿表面铜离子的活化,从而产生选择性抑制。
关键词:黄铜矿;闪锌矿;浮选分离;抑制剂;聚天冬氨酸
(Edited by Bing YANG)
Corresponding author: Wen-qing QIN, Tel: +86-731-88830884, E-mail: qinwenqing369@126.com
DOI: 10.1016/S1003-6326(21)65616-9
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
Abstract: Environmentally friendly flotation reagent, polyaspartic acid (PAPA), was tested as a potential selective depressant in the flotation separation of chalcopyrite and Cu-activated sphalerite. The depression mechanism of PAPA was revealed by contact angle measurements, Zeta potential measurements, Fourier transform infrared spectroscopy (FT-IR) analysis and inductively coupled plasma (ICP) measurement. The micro-flotation tests with single minerals showed that PAPA selectively depressed Cu-activated sphalerite, while chalcopyrite remained floatable. Moreover, a concentrate containing 31.40% Cu with a recovery of 92.43% was obtained in flotation tests of artificially mixed minerals. Results of contact angle measurements, Zeta potential measurements and FT-IR spectrum revealed that PAPA exerted a much stronger adsorption on Cu-activated sphalerite surface than on chalcopyrite surface, preventing the further adsorption of sodium diethyl dithiocarbamate (DDTC) on its surface. ICP measurements indicated that PAPA had an excellent complexing ability with Cu2+ in flotation pulp, weakening the activation of Cu species on sphalerite surface and producing selective depression.


