
J. Cent. South Univ. (2019) 26: 3534-3550
DOI: https://doi.org/10.1007/s11771-019-4271-8
Ilvaite as a thermodynamic recorder of multistage retrograde alteration in large Galinge skarn Fe deposit, western China
YU Miao(于淼)1, 2, Jeffrey M. DICK1, 2, MAO Jing-wen(毛景文)3, FENG Cheng-you(丰成友)3,
LI Bin(李斌)1, 2, LU An-huai(鲁安怀)1, 4, ZHU Yong-feng(朱永峰)4, LAI Jian-qing(赖健清)1, 2
1. Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring (Ministry of Education), Changsha 410083, China;
2. School of Geosciences and Info-Physics, Central South University, Changsha 410083, China;
3. MLR Key Laboratory of Metallogeny and Mineral Assessment, Institute of Mineral Resources,CAGS, Beijing 100037;
4. School of Earth and Space Sciences, Peking University, Beijing 100871, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract:
The ilvaite-bearing skarn associations in the Galinge skarn deposit were studied to determine their physicochemical formation conditions. A thermodynamic model setting pressure of 50 MPa (Pf=Ps=50 MPa) was set up to trace the skarn evolution. Petrographic evidence for replacement of garnet and magnetite by ilvaite in the early retrograde stage (Stage I) combined with thermodynamic modeling suggests that the alteration may have occurred at 400-470 °C under moderately high fO2 with △lgfO2(HM) ranges from -4 to -4.2. The model is based on a maximum pressure of 50 MPa calculated from magmatic amphibole geobarometer. The continuous breakdown of ilvaite with quartz to form ferro-actinolite and magnetite occur in the late retrograde stage (Stage II). The reactions occurred at 400-440°C under moderate fO2 (△lgfO2(HM): -4 to -4.4). In Stage III, the breakdown of ilvaite to form calcite, pyrite and ferroactinolite depends on XCO2 which can be estimated to be in a range of 0.005 to 0.05, and the reaction would occur at higher temperatures with increasing XCO2. Under these conditions, the breakdown occurs at 270-350 °C and low fO2 (△lgfO2(HM): up to -5.2). The thermodynamic model for continuous evolution from Stage I to Stage III completely records the conditions of the retrograde alteration, which is inconsistent with the thermobarometry imprints of fluid inclusions. Therefore, the petrography and phase relations of ilvaite are useful indicators of reaction conditions in various skarn deposit types.
Key words:
Galinge skarn deposit; ilvaite; retrograde alteration; thermodynamic properties;
Cite this article as:
YU Miao, Jeffrey M. DICK, MAO Jing-wen, FENG Cheng-you, LI Bin, LU An-huai, ZHU Yong-feng, LAI Jian-qing. Ilvaite as a thermodynamic recorder of multistage retrograde alteration in large Galinge skarn Fe deposit, western China [J]. Journal of Central South University, 2019, 26(12): 3534-3550.
DOI:https://dx.doi.org/https://doi.org/10.1007/s11771-019-4271-81 Introduction
Ilvaite, a mixed-valence Fe sorosilicate [1, 2], has long been recognized as an accessory gangue mineral in Fe, Zn and Pb-Zn skarn deposits [3-6]. The ilvaite group comprises the Mn-dominant endmember manganilvaite, ideally CaFe2+Fe3+(Mn, Fe2+)(Si2O7)O(OH)) and the Fe-dominant ilvaite, ideally (CaFe22+Fe3+(Si2O7)O(OH)) [7, 8]. The Mn-rich species occurs exclusively in marble- hosted distal Zn-(Pb) skarn deposits, and is closely associated with Mn-rich clinopyroxene (hedenbergite–johannsenite) ± rhodonite exoskarn minerals [5, 9]. In contrast, the Fe-rich species occurs in Fe, Au, Sn and W skarn deposits, and is associated with exoskarn minerals [10-12]. Ilvaite rarely occurs in the Cu skarn deposit, but it was found in Igarapé Bahia Cu-Au deposits which is a typical Fe-oxide Cu-Au-(U-REE) deposits in the Carajás region [13]. GRASER et al [14] reported that the ilvaite, epidote and garnet occur an endoskarn assemblage along fracture zones. Ilvaite is commonly considered as a retrograde alteration mineral in various skarn deposits [15-18], but FRANCHINI et al [12] suggested that ilvaite crystallizes on both prograde and retrograde paths. Nonetheless, since ilvaite is not present in large quantities in hydrothermal systems, its potential for recording hydrothermal ore-forming processes has long been underestimated.
This study documents the occurrence of ilvaite and the associated Ca-rich aluminosilicates from the Galinge Fe skarn deposit, and elucidates the physicochemical conditions of their formation and evolution. Mineral textures and stability relationships provide valuable information on their origin and relative timing of formation. In this study, substantial amounts of ilvaite geochemical data were compiled from various Fe, Zn ± Pb, Au, and Sn±W skarn deposits for comparing the ilvaite composition with the type of mineralization.
2 Geological background
The Galinge deposit in the Qinghai province, the largest Fe-polymetallic skarn deposit (1.8×108t) in western China, is located at the center of the boundary between the Qaidam Basin and the Qiman Tagh Orogen (Figure 1(a)) [19]. As the whole ore field is buried by the Quaternary in the basin, some characteristics of bed rocks and alteration are unclarified. The ore district is covered by Quaternary sediments at an average depth of 200 m. The Qiman Tagh metallogenic belt is one of the most prolific porphyry-skarn belts in western China [20-22], which extends from the city of Golmud in the east to the Altun strike-slip fault in the west (Figure 1(a)). The Qiman Tagh Terrane can be tectonically and chronologically separated into the North Qiman Tagh Terrane (NQT) and South Qiman Tagh Terrane (SQT), which are tectonically clipped by the Adatan fault in the east and Baiganhu fault in the west [23, 24] (Figure 1(a)). The NQT as a continent margin arc mainly develops abundant Paleozoic granitoids [25, 26]; and the SQT as an exotic terrane significantly carries Early Paleozoic oceanic island basalts [27], and develops Late Paleozoic and Early Mesozoic granitoids [28, 29]. Previous studies suggest that the single prolonged porphyry-skarn mineralization process was closely related to multiphase Middle- to Late-Triassic (237-204 Ma) granitic stocks or dyke intrusions in the SQT [22, 30, 31]. YU et al [32]distinguished two granitoid types in the belt, that is Early-stage (236-220 Ma) calc-alkaline granodiorite and monzogranite, and late-stage (219-204 Ma) syenogranite, and considered that the partial melting of the thickened lower crust and mantle- crustal mixing may be the primary petrogenetic mechanism for the voluminous Triassic Qiman Tagh intermediate to felsic intrusions [33, 34].
Exoskarn bodies at Galinge occur within the metamorphic aureoles of the granodiorite intrusion in the west [19]; thermal effects extend for approximately 1-1.5 km, and it is divided into six ore domains from the intrusive contacts (Figure 1(b)). Most of orebodies are located in the south of the mining area which is completely covered by Quaternary sediments in the basin. Therefore, some basic geological information is unavailable, especially in the north of the mining area where there is no magnetic anomaly and little prospecting has been done. The map shows a limestone unit that extends to the intrusion contact in the north, but this is a provisional interpretation based on limited geological information. The ore domains I and III are located within the xenoliths of the granodiorite intrusion. The ore domain II is located along the intrusive contact between the granodiorite and the serpentinization dolomitic marble, and the ore domains IV, V, and VI are distributed along the WNW-trending fracture zone within the exocontact zones. In the ore domains IV and V, the orebodies were mainly hosted in the limestone and basaltic andesite of the Cambrian- Ordovician Tanjianshan Formation. The limestone was intensively metamorphosed to marble before skarn formation. Magnetite is being mined, and Cu is produced as a byproduct of magnetite from these two ore domains. The ore domain VI hosts several small NW-trending orebodies, where galena, sphalerite and magnetite in the skarn (developed in the Tanjianshan Formation limestone) were mined.
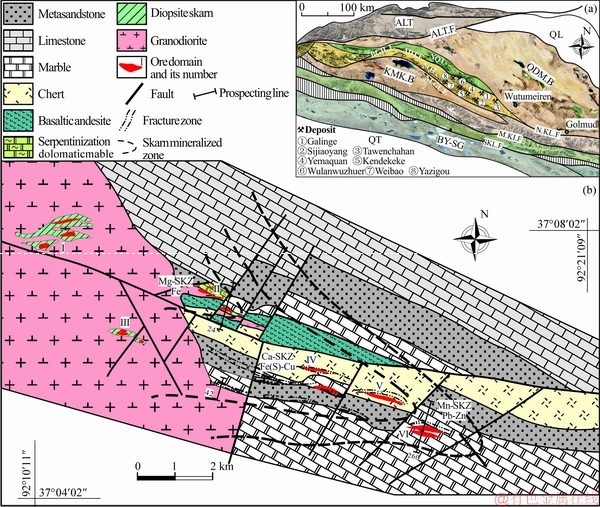
Figure 1 Satellite map of East Kunlun Orogen (a) and geological map of the Galinge skarn deposit (b) QL: Qilian terrane; BY-SG: Bayanhar-Songpan-Ganzi terrane; BGH.F.: Baiganhu Fault; ADT.F.: Adatan Fault; NLGL.F.: Nalinggelehe Fault; QDM.B.: Qaidam Basin; KMK.B.: Kumkule Basin; ALT.F.: Altun fault; S.KL.F.: South Kunlun Fault; M.KL.F.: Middle Kunlun Fault; N.KL.F.: North Kunlun Fault
Based on the development of variable skarn minerals in different ore domains, the Galinge skarn deposit displays zonation from the intrusive contact outwards (Figure 1(b)): Mg-rich skarn zonation (Mg-SKZ); Ca-rich skarn zonation (Ca-SKZ) developed with abundant Fe sulfides (e.g., pyrrhotite, pyrite and minor chalcopyrite); and Mn-Ca rich skarn zonation (Mn-SKZ) developed with galena and sphalerite [19]. The Mg-SKZ dominating ore domain II developed within dolomitic rocks nearest the pluton, and has lower sulfide content (6 vol%) and more abundant magnetite (20 vol%-35 vol%). The Mg-SKZ skarn forms narrow, zoned envelopes along the intrusive contacts with the dolomitic marble of the Tanjianshan Formation, and is also crosscut by later granodiorite. It was predominately composed of Mg-rich silicates which experienced intensive serpentinization. Moreover, the only significant Ca-bearing silicates are clinopyroxene and tremolite which are subordinate to forsterite. The Ca-Fe(S) skarn zonation including ore domains IV and V occurs distal to the contact, while magnetite disseminates elsewhere in Ca-Fe(S) skarn zonation. The Mn-Pb-Zn skarn zonation encompassing the ore domain VI occurs farthest from the contact and overlaps the Fe-zone, showing that skarn alteration reached its maximum extent in the distal part, the eastern ends of the deposit.
The Galinge skarn evolution includes the prograde-, retrograde- and quartz-calcite-sulfide stages (Figure 2). The temperature (T) is based on the thermobarometer of fluid inclustions from the Galinge skarns and intrusions. The prograde stage comprises an early sub-stage with wollastonite and olivine, and a late sub-stage with garnet, pyroxene and chondrodite. The retrograde alteration also comprises an early sub-stage with ilvaite, epidote, tourmaline, tremolite and hastingsite, and a late sub-stage with ferro-actinolite, axinite, chlorite, prehnite, phlogopite and serpentine. Calcite and quartz were formed throughout the retrograde stage. Magnetite mineralization mainly occurred in the retrograde stage, and ended with the beginning of the quartz-calcite-sulfide stage. Chalcopyrite, pyrrhotite, pyrite, galena, sphalerite, calcite, quartz and fluorite were formed in the quartz- calcite-sulfide stage.
The intrusions comprise mainly fine- to medium-grained granodiorite and quartz diorite, as well as diorite and porphyritic diorite dikes. The Galinge granodiorite was dated using zircon U-Pb ratios to be 229 Ma [35]. The quartz diorite was dated to be (234.4±0.6) Ma [36]. Phlogopite from the Galinge Mg-Fe skarn zone yielded a (234.2±3.5) Ma isochron age (40Ar/39Ar), which could be regarded as the mineralization age of magnetite [37].
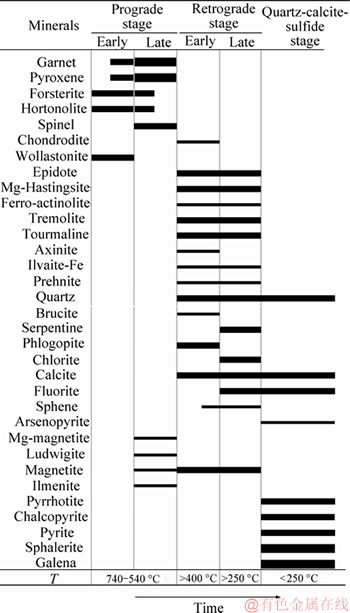
Figure 2 Paragenetic sequence of skarn minerals and mineralization at Galinge deposit
3 Sample characteristics and analytical methods
3.1 Sampling and petrography
The samples were collected from the drill core of ZK2901 at 215 m, and preliminarily termed chalcopyrite garnet amphibole skarns in the field. The specimen was selected to make polished thin sections for microscopic identification.
Ilvaite is a common accessory mineral that is typically formed in the retrograde stage in the Galinge exoskarn, and is mainly concentrated in the area between the ore domains II and IV. Ilvaite coexists with magnesio-hastingsite and magnetite (Figures 3(a) and (b)), and some andradite relics are locally kept in the system (Figure 3(e)). In this case, the rock shows inhomogeneous composition on the thin section scale, which reflects a disequilibrium association of andradite+magnetite+ilvaite. On the microprobe image of Figures 3(c) and (d), ilvaite occurs as spongy resinous masses or aggregates coexisting with ferro-actinolite, quartz and magnetite. Many grains of quartz, magnetite and ferro-actinolite fill the fissures of ilvaite, which indicates a disequilibrium association of ilvaite+ ferroactinolite+quartz+magnetite, and explains the formation of magnetite and ferroactinolite after ilvaite. Furthermore, ilvaite also occurs as euhedral prismatic crystals in calcite veins, and the ilvaite crystals are locally crosscut by later calcite (Figures 3(e) and (f)). In this case, calcite is interpreted to be entire of metamorphic origin, because of its sole occurrence as veins around ilvaite crystals. The rock appears to be compositionally inhomogeneous on thin-section scale, and the mineral association including ilvaite, calcite, ferroactinolite and magnetite do not show a mutual grain contact, which reflects a disequilibrium state (Figures 3(e) and (f)). The petrographic analysis gives a total of three different ilvaite-bearing replacement textures (Figure 3):1) andradite and magnetite replaced by ilvaite;2) ilvaite with quartz breakdown to ferro-actinolite and magnetite;3) ilvaite breakdown to calcite, ferroactinolite and pyrite. The formation of the three associations is completely documented in the retrograde alteration stage, suggesting that they record a continuous variation in temperature and oxidation state, although each texture shows strong replacement relationship.
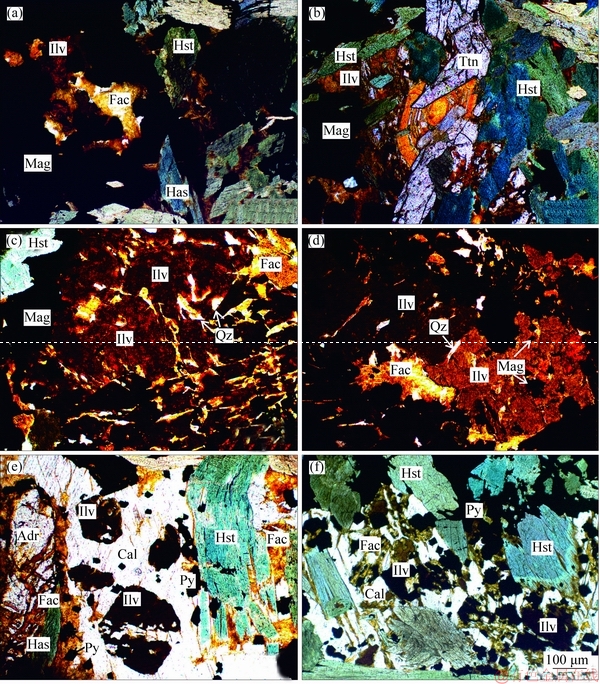
Figure 3 (a, b) Subhedral to anhedral scarlet ilvaite (Ilv) associated with hastingsite (Has), andradite (Adr), ferro-actinolite (Fac), titanite (Ttn) and magnetite (Mag), and locally enveloped by magnetite; (c, d) Anhedral ilvaite aggregates with magnetite and quartz inclusions, replaced by ferro-actinolite around the rims and fissures;(e, f) Dark brown subhedral to euhedral ilvaite in calcite (Cal)-pyrite (Py) veins, crosscut and filled by calcite along fissures, and replaced by ferro-actinolite around the rims
Under the microscope, magnesio-hastingsite is bluish-green to dark green, and forms anhedral to subhedral lamellae crystals. Replacement by ilvaite and ferro-actinolite is very common (Figures 3(a) and (b)). Pleochroic yellowish-green or bluish- green hastingsite is found intergrown with epidote or replacing garnet.
3.2 Analytical methods
Optical imaging was conducted to investigate the retrograde skarn mineral relations in ilvaite-bearing associations. These images were used to locate points for microanalysis of ilvaite and associated silicates. Electron microprobe analyses were conducted by a high resolution, highly stable SEM and a wavelength-dispersive/ energy-dispersive (WD/ED) combined JEOL JXA- 8230 electron probe microanalyzer (EPMA), at the MLR Key Laboratory of Metallogeny and Mineral Assessment, Institute of Mineral Resources, Chinese Academy of Geological Sciences, Beijing, China.
The facility has a tungsten source gun and is equipped with five wavelength-dispersive spectrometers (WDS) and one energy-dispersive spectrometer (EDS) featuring spectral imaging assures the most efficient and accurate analysis of data. The analytical deviation is 0.1%. It is placed in an environment with closely controlled temperature ((20±5) °C) and humidity (±4%). The accelerating potential was 15 kV; the probe current was 5-10 nA; the beam was focused to a spot size of 3-5 μm; counting time on the peak was 16 s for major elements; background counting times were half the peak counting time; and the typical total analysis time was 2-5 min. The raw data were corrected using the internal procedures of JEOL [38]. The detection limits and the typical average standard deviations for each elements depend on the error based on count statistics. The described configuration achieves detection limits for most elements down to 100×10-6 or lower at typical analytical conditions: acceleration voltage of -15 kV, beam current of 20 nA and counting time of 20 s. The back scattered electron (BSE) images were photographed under 15 kV accelerating voltage and 0.5 nA current beam. The analyses were calibrated using jadeite (Si, Na, and Al), forsterite (Mg), orthoclase (K), apatite (P), wollastonite (Ca), rutile (Ti), halite (Cl), and synthetic oxide (Cr, Mn, Fe, Ni) as standards.
The analyses are presented in Tables S1-S4 in Appendixs. Ilvaite, Ca(Fe2+,Fe3+)Fe2+Si2O7O(OH), a mixed-valence iron silicate, shows an ordered distribution of Fe2+ and Fe3+ on two non-equivalent octahedral sites [38]. The crystal structure consists of edge- sharing double chains of (Fe2+, Fe3+) octahedra (A site) running parallel to the e-axis; half as many larger Fe2+ octahedra (B site) are attached above and below the chains, sharing edges with the A site octahedra [2]. These chains are held together by the sorosilicate Si2O7 groups, and by Ca2+ ions in seven-fold coordination [39]. In order to calculate the stoichiometry of the ilvaites and associated minerals, and estimate the amounts of H2O, several assumptions were made. The structural formula was established assuming that the cation sum is 6 on the basis of 8.5 oxygen atoms. The proportion of H2O in ilvaite was calculated assuming 1 hydrogen atom per 9 oxygen atoms in the unit cell. This composition is close to the ideal ilvaite. Only small amount of Al3+, presumably replacing Fe3+ and Si4+, and a little Mn2+ and Mg2+ replacing Ca2+ and Fe2+ occur. These deviations from the ideal composition are typical of ilvaites from skarn deposits.
4 Mineral compositions
The chemical compositions of representative ilvaites from Stages I to III are very similar, and show a particularly Fe-rich character, close to the pure Fe-end-member (Figure 4). The Ca-Fe-Si ternary plot shows the composition of crystalline phases considered for stability calculations in the system Ca-Fe-Si-C-O-H, assuming that Al2O3, Na2O, H2O and O2 are saturated in the system. The shaded field shows the bulk composition for the stable relationship of ilvaite-bearing associations in the different stages. The solid black circle corresponds to the theoretical value of different minerals occurring in the ilvaite-bearing system. Mineral chemistry shows that the FeOtot content ranges from 46.03 wt% to 49.78 wt% (Tables S1- S4). The MnO content ranges from 0.21 wt% to 1.47 wt%. Mn2+-Fe2+ replacement unambiguously occurs at the M(2) site [8, 39]. Only small amount of Al2O3 are present, ranging from 0.21 wt% to 0.98 wt%, and the CaO content is between 13.62 wt% and 14.06 wt%.
The garnet coexisting with ilvaite is a grossular-andradite solid solution, containing moderate Al2O3 (8.65 wt%-9.76 wt%) and FeOtot (15.29 wt%-17.43 wt%) contents; the average composition is Adr55.2Gro42.9Pyr1.9.
Previous microprobe work distinguished two compositionally different but texturally identical secondary amphibole types, lying within the compositional fields for hastingsite and ferro- actinolite (Tables S1-S4) [41]. The associated hastingsite shows chlorine-rich character (Cl:0.89 wt%-1.58 wt%), a low Si content (5.89-6.27 a.p.f.u.) and XMg from 0.25 to 0.53. The ferro-actinolite replacing ilvaite shows a higher Si content than hastingsite, from 7.383 to 7.808 a.p.f.u. (Figure 4), and a wide XMg range of 0.25 to 0.48. The Cl content of ferro-actinolite is close to the detection limit. The FeOtot of magnetite ranges from 89.39 wt% to 93.38 wt% (Fe2O3: 62.89 wt%-68.23 wt%; FeO: 31.44 wt%-32.79 wt%) (Tables S1-S4).
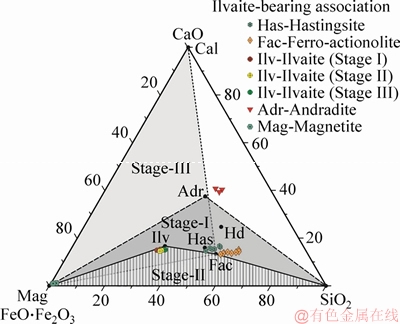
Figure 4 Compositions of ilvaite and associated minerals (andradite+hastingsite+ilvaite+ferroactinolite+magnetite) in SiO2-CaO-FeOtot ternary diagram
5 Discussion
5.1 Thermodynamic relations of ilvaite-bearing associations
Based on petrographic analysis, ilvaite occurs in three different stages based on the irreversible replacement of preexisting minerals followed by the breakdown of ilvaite (Figure 4). In Stage I, the garnet is altered to bluish-green amphibole (hastingsite), which is further altered into ilvaite. In Stage II, the ilvaite with SiO2 breaks down into ferro-actinolite and magnetite, and these product minerals can coexist in partial equilibrium, although ilvaite remains unstable through the reaction. In Stage III, the remaining ilvaite also replaced by ferro-actinolite and magnetite, but in this case calcite is also part of the secondary product. After the initial precipitation of magnetite, it is further altered to pyrite which indicates that there was a source of sulfide in the fluid.
Ilvaite is stable at medium to low temperatures (<450 °C) and high fluid pressures and occurs in a wide fO2 range because the Fe in ilvaite is not fully reduced [42]. MARTIN et al [43] considered that the replacement relationships of ilvaite requires that ilvaites unlikely form under pressures below 200 MPa, and shows that this mineral can only develop from fluids with low or very low CO2 content. However, we found that the activity of reacted minerals and H2O in the system also affects the stability of ilvaite, and the suitable geochemical conditions can yield ilvaite under low pressue of below 200 MPa. A lower PH2O/Ptotal also decreases the thermal stability range of ilvaite [42]. In addition, relatively low pH favors the formation of ilvaite [44]. However, the major temperature and oxidation state effects on ilvaite stability can be assessed by considering reactions between neutral species.
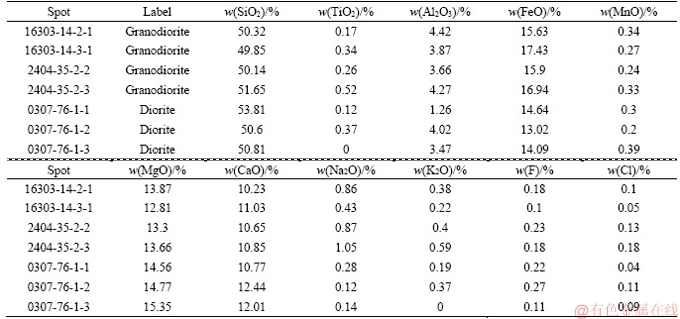
Figure 5 depicts the mineral stabilities calculated using thermodynamic data from BERMAN [45] supplemented with data for Adr, Fac, Hd, and Ilv [42]. The diagram is constructed for unit activity of mineral phases except for ilvaite (0.7), ferro-actinolite (0.8), and andradite (0.7). The line for the reaction in which CO2 is a reactant is drawn for three values of XCO2. The outer top x-axis shows the values of oxygen fugacity in the hematite-magnetite (HM) buffer; the fO2 scale on the y-axis is normalized with respect to the HM buffer [43]. Reactions involving Ilv are shown in bold; dotted red lines correspond to the indicated mineral buffers (QFM: quartz– fayalite–magnetite; NNO: nickel–nickel oxide). The arrow indicates the postulated trajectory of fO2 during skarn development. These estimates of activity are calculated with the ideal mixing approximation from representative mole fractions of the endmembers obtained from the microprobe analysis. The activity of H2O was set to 1. Values slightly lower than this are caused by mixing with CO2 and ionic species do not significantly affect the thermodynamic model. Grunerite was not included in these calculations because it was not identified in the Galinge deposit. Stable reactions in the system were identified using winTWQ [46], and then they were normalized to the hematite-magnetite buffer and plotted using CHNOSZ [47]. In the model, the lithostatic pressure, Ps, ranging based on the geobarometer calculation of magmatic amphibole from intrusions is 57-26 MPa (Table 2). Moreover, on the basis of hydrothermal fluid inclusion thermobarometry, the calculated hydrostatic pressure, Pf, is up to 40 MPa in the skarn stage [41]. In the subsequent discussion, an empirical pressure of Pf=Ps=50 MPa is assumed for the thermodynamic model, which is paralleled with that of most skarn deposits all over the word. In addition, the homogenization temperature, Th, of inclusions from different Galinge intrusions defines three populations [41], namely 500-560 °C, 380-460 °C, and 240-360 °C, corresponding to the skarn stage, early retrograde alteration stage, and late retrograde alteration stage, respectively.
In the model, although all the replacement reactions involve oxygen, and the coexisting minerals present in each of reactions serve to define oxygen fugacity, it is too little to result in detectable growth or breakdown of ilvaite. Therefore, The growth or breakdown of ilvaite either requires the presence of much more abundant oxidizing agent in place of dissolved oxygen, most likely sulphate, or is an open system accompanied by addition or removal of Fe, Ca or Si, or both.
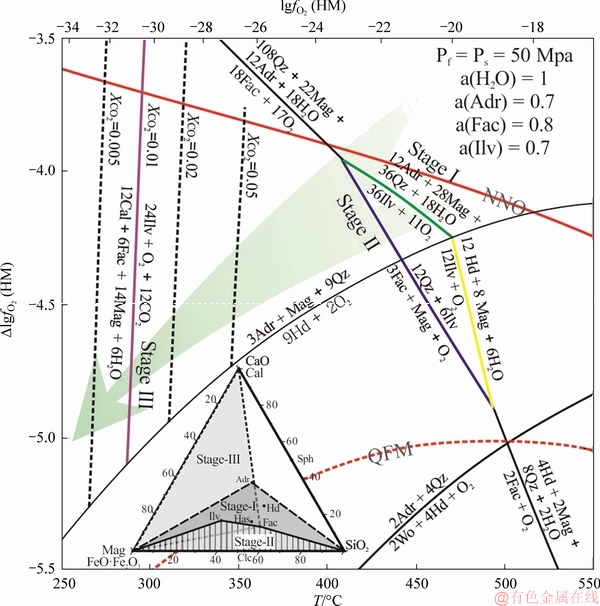
Figure 5 Temperatures-lgfO2 diagram for the Ca-Fe-Si-O-H-C system computed at Ps=50 MPa
Table 1 Geochemical and geophysical conditions of amphiboles from granodiorites and diorites in Galinge deposit
At high temperatures, and fO2 values between the HM buffer and hedenbergite stability field, formation of ilvaite occurs through the replacement of andradite and magnetite, and accompanied by the disappearance of quartz. In Stage I, ilvaite formed by the replacement reaction of 12Ca3Fe3+2Si3O12(andradite)+28Fe2+Fe3+2O4(magnetite)+36SiO2(quartz)+18H2O→36CaFe2+(Fe2+Fe3+)(Si2O7)·
O(OH)(ilvaite)+11O2 at 400-470 °C at a ΔlgfO2(HM) range of -4 to -4.2 (Figure 5). This reaction defines the maximum temperature for the formation of ilvaite in Stage I. Another reaction that could possibly form ilvaite is the breakdown of hedenbergite according to 6CaFe2+(Fe2+Fe3+)·
(Si2O7)O(OH)(ilvaite)+1/2O2→6CaFe2+Si2O6(hedenbergite)+4Fe2+Fe3+2O4(magnetite)+3H2O. However, we did not find any evidence for this reaction in the petrographic analyses. Therefore, the formation of ilvaite must be constrained to higher oxygen fugacities than that of the reaction of 3Adr+Mt+ 9Qtz→9Hd+2O2.
In order to interpret the metasomatic relationship, we have hypothesized that the associations formed by the breakdown of ilvaite are in partial equilibrium with fluid. The reaction corresponding to the breakdown of ilvaite to give ferro-actinolite and magnetite defines the low-T stability of ilvaite in Stage II (Figure 5). In Stage II, the replaced relationship corresponds to the reaction 6CaFe2+(Fe2+Fe3+)(Si2O7)O(OH)(ilvaite)+12SiO2(quartz)→3Ca2Fe2+5[Si8O22](OH)2(ferro-actinolite)+Fe2+Fe3+2O4(magnetite)+O2, which takes place at 400-440 °C, and a ΔlgfO2(HM) range of -4 to -4.4 (Figure 5).
Any ilvaite that remains after Stage II, is available for lower temperature of breakdown in Stage III. The breakdown of ilvaite at Stage III is tentatively related to the reaction 24CaFe2+(Fe2+Fe3+)(Si2O7)O(OH)(ilvaite) + 12CO2+O2→14Fe2+Fe3+2O4(magnetite)+12CaCO3(calcite) + 6Ca2Fe2+5[Si8O22](OH)2(ferro-actinolite)+6H2O (Figure 5) at ca. 270-350 °C under relatively low fO2 (<-4.5 ΔlgfO2(HM)). However, unlike the reaction in the preceding stages, this reaction is intensively affected by the activity of CO2 of the fluid (Figure 5). The temperature where this reaction occurs will increase from 270 to 350 °C when the XCO2 values are elevated from 0.005 to 0.05 (Figure 5), and the ΔlgfO2(HM) is up to-5.2.
To summarize the findings of the thermodynamic model, the ilvaite is a good recorder for the evolution of retrograde alteration stage. The successive change of temperature is in agreement with the imprint of fluid inclusions.
5.2 Comparison with other ilvaite-bearing skarn deposit types
Ilvaite is a common accessory mineral in various skarn- and vein-type deposits occurring mostly in Fe and Zn-Pb skarns, and rarely in Au and Sn-W skarns (Table 3). However, it rarely occurs in Cu skarn deposits, and it was only reported in a Cu-Au IOCG deposit and associated with skarns [13]. Ilvaite occurs typically as disseminated crystals, veins or aggregates in carbonates (mainly limestone) [18] and to a lesser degree in intermediate-mafic rocks [48, 49]. The formation of ilvaite has been attributed to either pneumatolytic alteration or hydrothermal replacement [14, 50]. In most cases, ilvaite has been regarded as a retrograde mineral. However, ilvaite from the Nikolaevsky Pb-Zn-Ag skarn deposit is exclusively formed in the prograde stage, and mainly occurs in a garnet–olivine–wollastonite–ilvaite–hedenbergite assemblage [51]. GRASER et al [14] suggest that the formation conditions of ilvaite in the Ilimaussaq complex, bearing the epidote+albite+andradite± prehnite assemblage, are ca. 500 °C and at a range of oxygen fugacities that includes the HM buffer. The model presented here also indicates that ilvaite may have crystallized in a wide temperature range (400-470 °C), but at an oxygen fugacity below the HM buffer and above NNO buffer (Figure 5) in Stage I.
The variable composition of ilvaite makes it potentially useful for indicating the geochemical environment of formation [12]. The chemistry of ilvaite is deeply influenced by the host rocks and magmatic-hydrothermal fluids, together with mineral composition of the prograde stage skarn [52]. Therefore, it is necessary to compare the studied ilvaite with that of the other skarn deposits.The ilvaite compositions from various skarn deposits are plotted in Figure 6, and associated references for all presented data are listed in Table 3. The ilvaite composition diagram for various important skarn deposits in the world shows that the data points fall into distinctive fields (Figure 6).
Table 3 Selected ilvaite-bearing skarn and vein type deposits, and a IOCG deposit
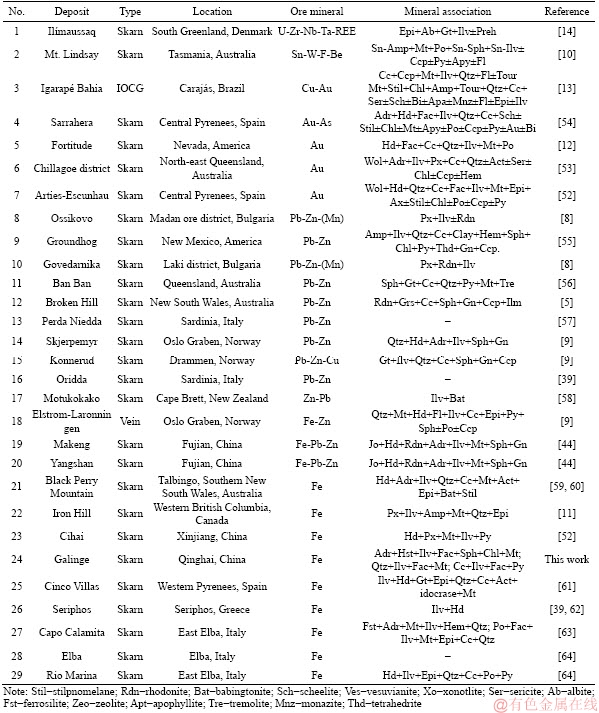
Ilvaite from Fe skarn deposits is compositionally different from the ilvaite from Au, Fe-Pb-Zn, Pb-Zn and Sn skarn deposits. It has higher Mg than that from Au deposits, and lower Mn than the Pb-Zn and Fe-Pb-Zn skarns (Figure 6(a)). Ilvaite from Sn skarns has the lowest Fe3+ content among all skarn types, with total Fe contents intermediate between that of Zn and Au-Fe skarn deposits (Figure 6(b)). We suppose that the varying Fe3+/Fe2+ ratios from Sn to Au skarn deposits is probably due to an increase in the fO2 of fluids. Most ilvaite from Fe-skarn deposits occurs in the skarn replacement of limestone, and grows simultaneously with amphibole and magnetite, as a replacement of Fe-rich pyroxene. Generally, ilvaite compositions in Fe skarn deposits are more Fe-rich than those of Zn-Pb deposits that are Mn-rich (Figure 6(a)). The varying Fe-Mn content of ilvaite from Fe to Pb-Zn skarns may be as a consequence of a decrease in temperature (Figure 6(a)). In addition, most MgO in ilvaite was suggested to be derived from the alteration of the prograde-stage Mg-bearing silicates [52], which usually takes place at ca. 450 °C under low pressure [65]. This temperature is near the stability limit for ilvaite (Figure 5).
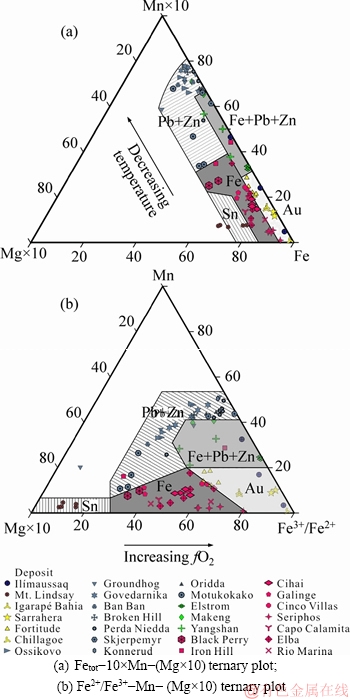
Figure 6 Ternary plot of microprobe analyses of ilvaite from various skarn deposits:
Mn-rich members of the ilvaite-manganilvaite solid solution occur in the distal parts of Pb-Zn skarn deposits [8, 43], and appear as: 1) radiating crystals or nets among the interstices of pyroxene aggregates; 2) aggregates or nets in wollastonite along the skarn-marble front; 3) along fine quartz-calcite veins enveloped by pyroxene or rhodonite aggregates. Generally, the retrograde alteration started with the occurrence of ilvaite. In some cases, the clinopyroxene and andradite skarn is crosscut by calcite-quartz-ilvaite veins [53]. LOGAN [66] reported quartz fluid inclusion data from the ilvaite-quartz-calcite retrograde assemblage in the Gualilan Zn-Pb-(Ag-Au) skarn deposit, in which temperatures and salinities range from 237 to 251 °C and 1.4 wt% to 2 wt% NaCl eq., respectively. Based on these observations, ilvaite has a broad stability field from 440 to 230 °C. In addition, BONEV et al [8] argued that the presence of manganilvaite in some deposits reflects the evolution of the hydrothermal environment from a decrease to a more oxidized and hydrated conditions. However, a trend toward relatively reducing conditions at lower temperature is consistent with the petrographic and compositional observations in this study (Figure 5).
Very rarely, ilvaite also occurs in hydrothermal veins as an accessory mineral [9]. For instance, at the Fossum Fe ore field (in the Oslo Rift near Skien), ilvaite occurs in hydrothermal veins (quartz+magnetite±fluorite±hedenbergite±andradite±ilvaite±calcite) that intruded the Proterozoic granitic gneisses [9]. The ilvaite from the hydrothermal veins contains generally much higher Mn than those from massive Au and Sn skarn deposits, also similar to some ilvaite components from Fe skarn [67].
Comparisons of electron microprobe analyses demonstrate that the ilvaite from Au-skarn deposits have higher Fe and lower Mn than Pb-Zn skarns, which is compositionally similar to ilvaite from Fe-skarn deposits [12] (Figure 6). However, the Au-skarn ilvaite contains lower MgO than its Fe-skarn counterpart (Figure 6). Ilvaite in Au-skarn deposits generally occurs in both breccia groundmass and veins as subhedral crystals [12]. Observed textures in the Fortitude Au skarn suggest that ilvaite formed during the breakdown of iron-rich pyroxene in magnetite-rich exoskarn, similar to the ferroactinolite, or replaced calcite grains at the marble front [12], probably suggesting an origin from the reaction: Fe2+Fe3+2O4(magnetite)+ CaCO3(calcite)+2SiO2(quartz)+1/2H2O=CaFe2+(Fe2+Fe3+)(Si2O7)O(OH)(ilvaite)+CO2+1/4O2. The ilvaite from the Cavnic deposit was found enveloping native gold, and native gold forming a little veins on the fine crack in the ilvaite, which reflects that the ilvaite crystallization took place at ca 400 °C before gold formation [67]. The presence of coeval rhodonite further constrains the temperature to be higher than 400 °C, because synthesis experiments show rhodonite to be stable in the presence of water only at ca. 425-450 °C at 300 MPa [68, 69].
In Arties-Escunhau Au skarn deposits, Ilvaite is formed before ferro-actinolite, according to the reaction of 10CaFe2+(Fe2+Fe3+)(Si2O7)O(OH)(ilvaite)+4CO2+4SiO2(quartz)=4CaCO3(calcite)+3Ca2Fe2+5[Si8O22](OH)2(ferro-actinolite)+5Fe2+Fe3+2O4(magnetite)+2H2O [54]. However, DUNKEL [65] suggested that ilvaite may have formed by replacing ferro-actinolite in the Capo Calamita skarn deposit. This reaction, which corresponds to Stage III in this study, allows the formation of ilvaite to proceed at temperatures as low as 270 to 350 °C, depending on the XCO2 (Figure 5).
6 Conclusions
1) The Galinge skarn followed a decreasing
temperature path that comprises a prograde- and retrograde alteration stages, and quartz-calcite- sulfide stage. The prograde stage shows a high temperature (>500 °C) and a more oxidizing environment (above the NNO oxygen buffer).
2) In terms of the formation and breakdown of ilvaite, it shows intensive replacement textures. The garnet + ilvaite + magnetite association was formed at a relatively high temperature range of ca. 400- 470 °C, and under an fO2 of approximately -4 – -4.2 log units below hematite-magnetite, corresponding to the Stage I conditions. The ferro- actinolite+ilvaite+quartz+magnetite association was formed in Stage II at a medium temperature (400-440 °C) and under a ΔlgfO2(HM) -4 to -4.4. Moreover, the ilvaite stability field extends to more reducing conditions (up to -5.2 ΔlgfO2(HM)), at a lower temperature range (ca. 275-375 °C) in the Stage III. In this stage, the activity of CO2 significantly affects the stability of ilvaite.
3) Although there are some similarities in minerals and hydrothermal fluid conditions of various skarn deposit types, the major differences in the compositions of ilvaite could arise from subtle changes of fO2, temperature, fluid components and prograde silicates composition.
Appendix
Table S1 Electron microproble analyses of Hst and Fac based on 15 cations and 23 oxygen atoms
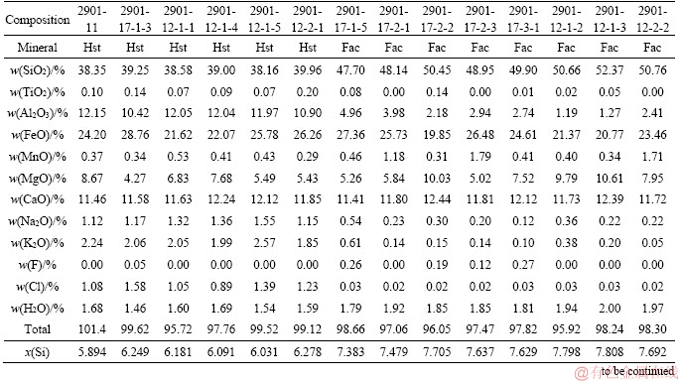
Continued
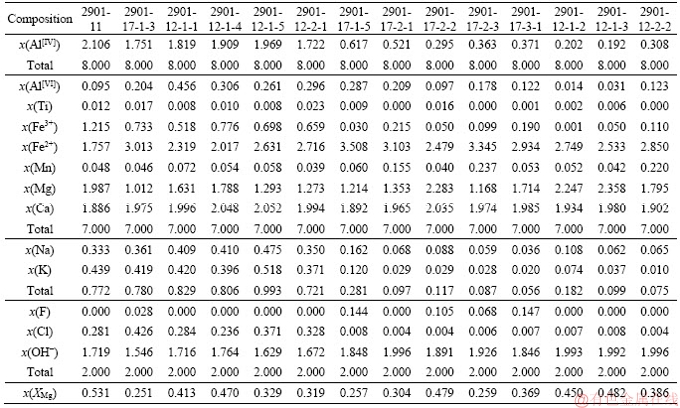
Table S2 Electron microproble analyses of Adr
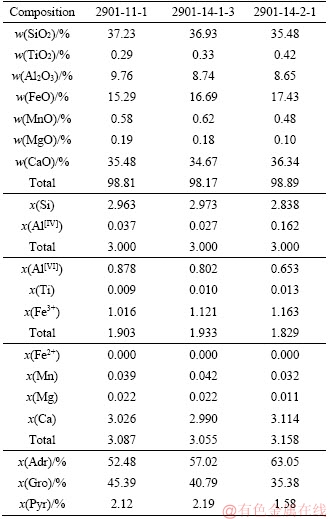
Table S3 Electron microproble analyses of Mt
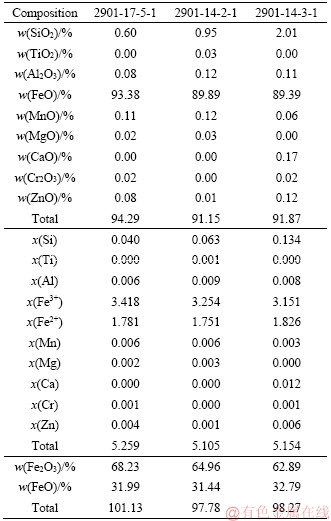
Table S4 Electron microproble analyses of Ilv based on 6 cations and 8.5 oxygen atoms
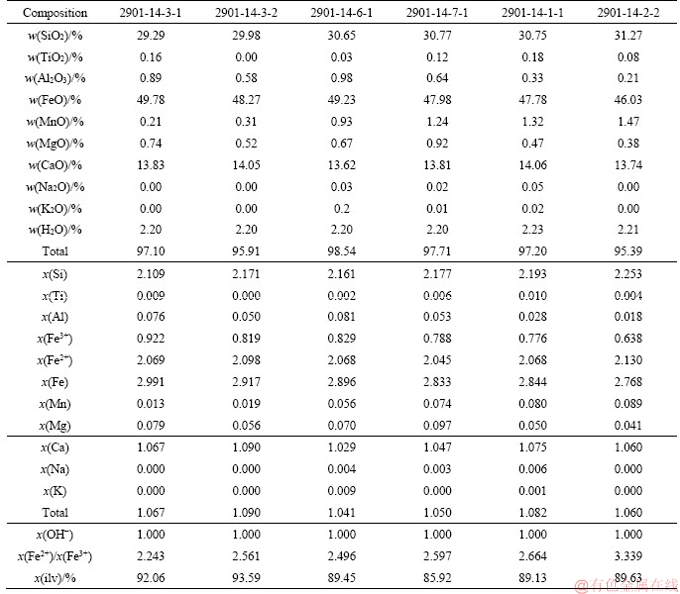
References
[1] GHOSE S, SEN GUPTA P K, SCHLEMPER E O. Electron ordering in ilvaite, a mixed-valence iron silicate: Crystal structure refinement at 138 K [J]. American Mineralogist, 1985, 70: 1248-1252.
[2] GHOSE S, TSUKIMURA K, HATCH D. Phase transitions in ilvaite, a mixed-valence iron silicate [J]. Physics and Chemistry of Minerals, 1989, 16(5): 483-496.
[3] BARTHOLOME P, DIMANCHE F. On the paragenesis of ilvaite in Italian skarns [J]. Ann Soc Geol Belg, 1967, 90: 533-565.
[4] BURT D M. Multisystems analysis of the relative stabilities of babingtonite and ilvaite [J]. Carnegie Inst Wash Year Book, 1971, 70: 189-197.
[5] PLIMER I R, ASHLEY P M. Manganoan ilvaite from broken hill, N.S.W. and Ban Ban, Queensland, Australia [J]. Mineralogical Magazine, 1978, 42(321): 85-88.
[6] EINAUDI M T, BURT D M. A special issue devoted to skarn deposits (Introduction; terminology, classification, and composition of skarn deposits) [J]. Economic geology, 1982, 77(4): 745-754.
[7] VASSILEVA R D, BONEV I K, ZOTOV N. High-Mn ilvaites from the skar Pb-Zn deposits in the Central Rhodopes [C]// Mineral Deposits at the Beginning of the 21st Century, 2001: 925-928.
[8] BONEV I K, VASSILEVA R D, ZOTOV N, KOUZMANOV K. Manganilvaite, CaFe2+Fe3+(Mn, Fe2+)(Si2O7)O(OH), a new mineral of the ilvaite group from Pb-Zn skarn deposits in the Rhodope Moutntains, Bulgaria [J]. The Canadian Mineralogist, 2005, 43(3): 1027-1042.
[9] LARSEN A O, DAHLGREN S. Ilvaite from the Oslo graben, Norway [J]. Neues Jahrbuch Für Mineralogie - Monatshefte, 2002, 4: 169-181.
[10] KWAK T A P. The geology and geochemistry of the zoned, Sn-W-F-Be skarns at Mt. Lindsay, Tasmania, Australia [J]. Economic Geology, 1983, 78(7): 1440-1465.
[11] MEINERT L D. Mineralogy and petrology of iron skarns in Western British Columbia, Canada [J]. Economic Geology, 1984, 79(5): 869-882.
[12] FRANCHINI M B, MEINERT L D, VALLES J M. First occurrence of ilvaite in a gold skarn deposit [J]. Economic Geology, 2002, 97(5): 1119-1126.
[13] TALLARICO F H B. Occurrence of ilvaite in the Igarapé Bahia Cu-Au deposit, Carajás Province, Brazil [J]. Revista Brasileira de Geociências, 2002, 32(1): 149-152.
[14] GRASER G, MARKL G. Ca-rich ilvaite-epidote-hydrogarnet endoskarns: a record of late-magmatic fluid influx into the persodic ilimaussaq complex, South Greenland [J]. Journal of Petrology, 2007, 49(2): 239-265.
[15] EINAUDI M T, MEINERT L D, NEWBERRY R J. Skarn deposits [J]. Economic Geology, 1981, 75: 317-391.
[16] MEINERT L D. Skarns and skarn deposits [J]. Geoscience Canada, 1992, 19(4): 145-162.
[17] MEINERT L D. Igneous petrogenesis and skarn deposits [J]. Mineral Deposit Modeling, 1993, 40: 569-583.
[18] MISRA K C. Understanding mineral deposits [M]. Netherlands: Springer, 2000.
[19] YU M, FENG C Y, BAO G Y, LIU H C, ZHAO Y M, LI D X, XIAO Y, LIU J N. Characteristics and zonation of skarn minerals in Galinge iron deposit, Qinghai Province [J]. Mineral Deposits, 2013, 32(1): 55-76.
[20] SHE H Q, ZHANG D Q, JING X Y, GUAN J, ZHU H P, FENG C Y, LI D X. Geological characteristics and genesis of the Ulan Uzhur porphyry copper deposit in Qinghai [J]. Geology in China, 2007, 34(2): 306-314. (in Chinese)
[21] LIU J N, FENG C Y, ZHAO Y M, LI D X, XIAO Y, ZHOU J H, MA Y S. Characteristics of intrusive rock, metasomatites, mineralization and alteration in Yemaquan skarn Fe-Zn polymetallic deposit, Qinghai Province [J]. Mineral Deposits, 2013, 32(1): 77-93.
[22] ZHAO Y M, FENG C Y, LI D X, LIU J N, XIAO Y, YU M, MA S C. Metallogenic setting and mineralization-alteration characteristics of major skarn Fe-polymetallic deposits in Qimantag area, western Qinghai Province [J]. Mineral Deposits, 2013, 32(1): 1-19.
[23] YU M, FENG C Y, SANTOSH M, MAO J W, ZHU Y F, ZHAO Y M, LI D X, LI B. The qiman tagh orogen as a window to the crustal evolution in northern Qinghai-Tibet plateau [J]. Earth-Science Reviews, 2017, 167: 103-123.
[24] WANG B Z, LUO Z H, LI H Y, CHEN H W, HU X L. Petrotectonic assemblages and temporal-spatial framework of the Late Paleozoic-Early Mesozoic intrusions in the Qimantage Corridor of the East Kunlun belt [J]. Geology in China, 2009, 36(4): 769-782. (in Chinese)
[25] LI W, NEUBAUER F, LIU Y, GENSER J, REN S, HAN G, LIANG C. Paleozoic evolution of the Qimantagh magmatic arcs, Eastern Kunlun Mountains: constraints from zircon dating of granitoids and modern river sands [J]. Journal of Asian Earth Sciences, 2013, 77: 183-202.
[26] WANG C, LIU L, XIAO P, CAO Y, YU H, MEERT J G, LIANG W. Geochemical and geochronologic constraints for Paleozoic magmatism related to the orogenic collapse in the Qimantagh–South Altyn region, northwestern China [J]. Lithos, 2014, 202: 1-20.
[27] MENG F, CUI M, WU X, REN Y. Heishan mafic-ultramafic rocks in the Qimantag area of Eastern Kunlun, NW China: remnants of an Early Paleozoic incipient island arc [J]. Gondwana Research, 2015, 27(2): 745-759.
[28] CHEN J, WANG B, LI B, ZHANG Z, QIAO B, JIN T. Zircon U-Pb ages, geochemistry, and Sr-Nd-Pb isotopic compositions of Middle Triassic granodiorites from the Kaimuqi area, East Kunlun, Northwest China: Implications for slab breakoff [J]. International Geology Review, 2015, 57(2): 257-270.
[29] YAO L, LU Z C, ZHAO C S, PANG Z S, YU X F, ZHU X Y, LI Y S, LIU P, LI S T, ZHANG M C. Geochronological study of granitoids from the Niukutou and B section of the Kaerqueka deposits, Qimantag area, Qinghai Province: Implications for Devonian magmatism and mineralization [J]. Geological Bulletin of China, 2016, 35(7): 1158-1169. (in Chinese)
[30] FENG C Y, LI D S, WU Z S, LI J H, ZHANG Z Y, ZHANG A K, SHU X F, SU S S. Major types, time-space distribution and metallogenesis of polymetallic deposits in the Qimantage metallogenic belt, eastern Kunlun area [J]. Northwestern Geology, 2010, 43(4): 10-17.
[31] MAO J W, ZHOU Z H, FENG C Y, WANG Y, ZHANG C, PENG H, MIAO Y. A preliminary study of the Triassic large-scale mineralization in China and its geodynamic setting [J]. Geology in China, 2012, 39(6): 1437-1471. (in Chinese)
[32] YU M, FENG C Y, ZHAO Y M, LI D X. Genesis of post-collisional calc-alkaline and alkaline granitoids in Qiman Tagh, East Kunlun, China [J]. Lithos, 2015, 239: 45-59.
[33] LUO Z H, KE S, CAO Y Q, DENG J F, ZHAN H W. Late Indosinian mantle-derived magmatism in the East Kunlun [J]. Geological Bulletin of China, 2002, 21(6): 292-297. (in Chinese)
[34] LIU C, MO X, LUO Z, YU X, CHEN H, LI S, ZHAO X. Mixing events between the crust-and mantle-derived magmas in Eastern Kunlun: Evidence from zircon SHRIMP II chronology [J]. Chinese Science Bulletin, 2004, 49(8): 828-834. (in Chinese)
[35] YU M. Geochemistry and zonation of the Galinge iron deposit, Qinghai province [D]. Beijing : China University of Geosciences (Beijing), 2013. (in Chinese)
[36] GAO Y, LI W, MA X. Genesis, geochronology and Hf isotopic compositions of the magmatic rocks in Galinge iron deposit, eastern Kunlun [J]. Journal of Lanzhou University (Natural Sciences), 2012, 48(2): 36-47. (in Chinese)
[37] YU M, FENG C, LIU H, LI D, ZHAO Y, LI D, LIU J, WANG H, ZHANG M. 40Ar-39Ar geochronology of the Galinge large skarn iron deposit in Qinghai province and geological significance [J]. Acta Geologica Sinica, 2015, 89(3): 510-521.
[38] ARMSTRONG J T. Quantitative elemental analysis of individual microparticles with electron beam instruments [M]. Electron Probe Quantitation. Boston, MA: Springer US, 1991.
[39] CARROZZINI B. Crystal structure refinements of ilvaite: new relationships between chemical composition and crystallographic parameters [J]. European Journal of Mineralogy, 1994, 6(4): 465-480.
[40] TAKEUCHI Y, SAWADA H, TANIGUCHI H. The ilvaite problem [J]. The Institute of Natural Sciences Nihon University, 1993, 28: 39-43.
[41] YU M, FENG C Y, ZHU Y F, MAO J W, ZHAO Y M, LI D X. Multistage amphiboles from the Galinge iron skarn deposit in Qiman Tagh, western China: Evidence of igneous rocks replacement [J]. Mineralogy and Petrology, 2017, 111(1): 81-97.
[42] GUSTAFSON W I. The stability of andradite, hedenbergite, and related minerals in the system Ca: Fe: Si: O: H [J]. Journal of Petrology, 1974, 15(3): 455-496.
[43] MARTIN J, DELGADO SOLERI GIL A. Ilvaite stability in skarns from the northern contact of the Maladeta batholith, central Pyrenees (Spain) [J]. European Journal of Mineralogy, 2010, 22(3): 363-380.
[44] ZHAO Y M, TAN H J, XU Z N, YUAN R G, BI C, ZHENG R L, LI D X, SUN J H. Makeng type calcic skarn iron deposit in the Southwest of Fujian province [M]. Beijing: Bulletin of the institute of mineral deposit, Chinese Academy of Geological Sciences, 1983.
[45] BERMAN R G. Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO- Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2 [J]. Journal of Petrology, 1988, 29(2): 445-522.
[46] BERMAN R G. WinTWQ (version 2.3): A software package for performing internally-consistent thermobarometric calculations [R]. Natural Resources Canada/ESS/Scientific and Technical Publishing Services, 2007.
[47] DICK J M. Calculation of the relative metastabilities of proteins using the CHNOSZ software package [J]. Geochemical Transactions, 2008, 9(1): 1-17.
[48] BARTON M, van BERGEN M J. Secondary ilvaite in a dolerite dyke from Rogaland, SW Norway [J]. Mineralogical Magazine, 1984, 48(348): 449-456.
[49] WANG Y S. Analysis on special case of ilvaite enrichment in a certain iron deposit [J]. Qinghai Geology, 1994, 3(2): 19-20.
[50] PETERSEN O V, MICHEELSEN H I, LEONARDSEN E S. Bavenite, Ca4Be3Al[Si9O25(OH)3], from the Ilímaussaq alkaline complex, South Greenland [J]. Neues Jahrbuch Für Mineralogie, Monatsheft, 1995, 7: 321-325.
[51] ROGULINA L I, SVESHNIKOVA O L. The Nikolaevsky base-metal skarn deposit, Primorye, Russia [J]. Geology of Ore Deposits, 2008, 50(1): 60-74.
[52] TANG P Z, WANG Y W, WANG J B, LONG L L, ZHANG H Q, LIAO Z. Finding and Significance of Ilvaite in the Cihai Iron Deposit, Xinjiang Autonomic Region, China [J]. Acta Mineralogica Sinica, 2011, 31(1): 9-16.
[53] LEHRMANN B. Polymetallic mineralisation in the Chillagoe district of north-east Queensland: Insights into base metal rich intrusion-related gold systems [D]. Townsville: James Cook University, 2012.
[54] MARTIN J, DELGADO SOLERI GIL A. Ilvaite stability in skarns from the northern contact of the Maladeta batholith, Central Pyrenees (Spain) [J]. European Journal of Mineralogy, 2010, 22(3): 363-380.
[55] MEINERT L D. Skarn zonation and fluid evolution in the Groundhog mine, Central mining district, New Mexico [J]. Economic Geology, 1987, 82(3): 523-545.
[56] ASHLEY P M. Geology of the Ban Ban zinc deposit, a sulfide-bearing skarn, southeast Queensland, Australia [J]. Economic Geology, 1980, 75(1): 15-29.
[57] BONAZZI P, BINDI L. Structural properties and heat- induced oxidation-dehydrogenation of manganoan ilvaite from Perda Niedda mine, Sardinia, Italy [J]. American Mineralogist, 2002, 87(7): 845-852.
[58] BRATHWAITE R L, ISAAC M J, CHALLIS G A, BROOK F J. Tertiary limestone and Zn-Pb mineralised skarn at Motukokako, Cape Brett, northern New Zealand [J]. Journal of the Royal Society of New Zealand, 1990, 20(4): 427-438.
[59] GOLE M J. Ca-Fe-Si skarns containing babingtonite: first known occurrence in [J]. The Canadian Mineralogist, 1981, 19: 269-277.
[60] GOLE M J. Iron calc-silicate rocks at black perry mountain, Talbingo, Southern New South Wales [M]. Sydney: Macquarie University, 1972.
[61] PESQUERA A, VELASCO F. An occurrence of ilvaite layers in the Cinco villas metasomatic rocks, Western Pyrenees (Spain) [J]. Mineralogical Magazine, 1986, 50(358): 653-656.
[62] SALEMINK J. Skarn and ore formation at Seriphos, Greece as a consequence of granodiorite intrusion [D]. Utrecht: Utrecht University, 1985.
[63] DUNKEL I. The genesis of east Elba iron ore deposits and their interrelation with Messinian tectonics [D]. Baden-Waerttemberg: Universitat Tübingen, 2002.
[64] SCHIENER A. Lievrit von Seriphos [J]. Zeitschrift Für Kristallographie-Crystalline Materials, 1933, 85(1-6): 89-118.
[65] VERKAEREN J, BARTHOLOME P. Petrology of the San Leone magnetite skarn deposit (S.W. Sardinia) [J]. Economic Geology, 1979, 74(1): 53-66.
[66] LOGAN M A V. Mineralogy and geochemistry of the Gualilán skarn deposit in the Precordillera of western Argentina [J]. Ore Geology Reviews, 2000, 17(1, 2): 113-138.
[67] HIRTOPANU P, ANDERSEN J C, HARTOPANU I, UDUBASA S S. Ilvaite from the Cavnic deposit, Romania [J]. Romanian Journal of English Studies, 2012: 62-65.
[68] MARESCH W V, MOTTANA A. The pyroxmangite- rhodonite transformation for the MnSiO3 composition [J]. Contributions to Mineralogy and Petrology, 1976, 55(1): 69-79.
[69] MOMOI H. Hydrothermal crystallization of MnSiO3 polymorphs [J]. Mineralogical Journal, 1974, 7(4): 359-373.
(Edited by ZHENG Yu-tong)
中文导读
黑柱石-青海尕林格矽卡岩铁矿多期退化蚀变记录
摘要:尕林格大型矽卡岩铁矿位于祁漫塔格斑岩-矽卡岩成矿带内,发育一套典型的Ca质退化蚀变矽卡岩系列。其中,含黑柱石退化蚀变组合可以很好地反演流体交代热动力学过程。岩石学证据表明在早期退化蚀变阶段(Stage I),黑柱石交代石榴子石和磁铁矿,该反应根据岩浆角闪石地质温压计设定最大压力条件50 MPa,获得热动力学T=400~470 °C和△lgfO2(HM)=-4~-4.2;在晚期退化蚀变阶段(Stage II),黑柱石和石英反应生成磁铁矿和铁阳起石,该反应发生在400~440°C和△lgfO2(HM)=-4~ -4.4热动力学条件范围内;到了方解石-石英-硫化物阶段(Stage III),黑柱石分解形成方解石、黄铁矿和铁阳起石,该反应发生温度随着XCO2的升高而升高,在XCO2=0.005-0.05范围内,分解反应条件为T=270-350°C和△lgfO2(HM)<-5.2。随着黑柱石的连续演化,该热动力学模型完整地记录了退化蚀变反应的发生过程。除此之外,流体体系中Fe和Mg的含量会强烈影响黑柱石在矽卡岩体系中的稳定性。因此,黑柱石的岩石学和相变关系可以很好地指示不同类型矽卡岩矿床的交代反应过程。
关键词:尕林格矽卡岩矿床;黑柱石;退化蚀变;热动力学属性
Foundation item: Projects(41172076, 41802080) supported by the National Natural Science Foundation of China; Project(1212011085528) supported by Geological Survey Program from the China Geological Survey; Project(2019CX035) supported by Innovation-driven Plan of Central South University, China; Project(201411025) supported by the Scientific Research Fund from Ministry of Land and Re-sources, China; Project(201309) supported by the Program of High-level Geological Talents, China; Project(201112) supported by the Youth Geological Talents of the China Geological Survey
Received date: 2019-01-13; Accepted date: 2019-10-22
Corresponding author: FENG Cheng-you, PhD, Researcher; E-mail: fengchy@cags.ac.cn; ORCID: 0000-0001-6706-8681; LI Bin, PhD, Associated Professor; Tel: +86-18007489630; E-mail: cutelb@csu.edu.cn; ORCID: 0000-0001-6918-0749
Abstract: The ilvaite-bearing skarn associations in the Galinge skarn deposit were studied to determine their physicochemical formation conditions. A thermodynamic model setting pressure of 50 MPa (Pf=Ps=50 MPa) was set up to trace the skarn evolution. Petrographic evidence for replacement of garnet and magnetite by ilvaite in the early retrograde stage (Stage I) combined with thermodynamic modeling suggests that the alteration may have occurred at 400-470 °C under moderately high fO2 with △lgfO2(HM) ranges from -4 to -4.2. The model is based on a maximum pressure of 50 MPa calculated from magmatic amphibole geobarometer. The continuous breakdown of ilvaite with quartz to form ferro-actinolite and magnetite occur in the late retrograde stage (Stage II). The reactions occurred at 400-440°C under moderate fO2 (△lgfO2(HM): -4 to -4.4). In Stage III, the breakdown of ilvaite to form calcite, pyrite and ferroactinolite depends on XCO2 which can be estimated to be in a range of 0.005 to 0.05, and the reaction would occur at higher temperatures with increasing XCO2. Under these conditions, the breakdown occurs at 270-350 °C and low fO2 (△lgfO2(HM): up to -5.2). The thermodynamic model for continuous evolution from Stage I to Stage III completely records the conditions of the retrograde alteration, which is inconsistent with the thermobarometry imprints of fluid inclusions. Therefore, the petrography and phase relations of ilvaite are useful indicators of reaction conditions in various skarn deposit types.


