
Comparative study on biosorption of Pb(Ⅱ) and Cr(Ⅵ) by Synechococcus sp.
SHEN Li(申 丽), XIA Jin-lan(夏金兰), HE Huan(何 环), NIE Zhen-yuan(聂珍媛)
Key Laboratory of Biometallurgy of Ministry of Education,
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 20 September 2008; accepted 5 November 2008
Abstract:
The comparative study on adsorptions of Pb(Ⅱ) and Cr(Ⅵ) ions by free cells and immobilized cells of Synechococcus sp. was performed, in which different aspects including Zeta potential of the cells, the influence of pH, temperature and initial concentration of metal ions, as well as adsorption kinetics and mechanism were referred. The lyophilized free cells have a surface isoelectric point at pH 3, and the correlative experiment indicates that there is an electrostatic adsorption feature of Cr(Ⅵ) and Pb(Ⅱ). The immobilization of the free cells by Ca-alginate does not significantly modify the adsorption features of the biosorbent. The absorption processes of Cr(Ⅵ) and Pb(Ⅱ) on both free and immobilized cells are apparently affected by pH and the initial concentration of metal ions in the bulk solution, but are much weakly affected by temperature in the test range of 10-50 ℃. The slow course of biosorption follows the first order kinetic model, the adsorption of Pb(Ⅱ) obeys both Langmuir and Freundlich isotherm models, while the adsorption of Cr(Ⅵ) obeys only Freundlich model. FT-IR results indicate that carboxylic, alcoholic, amide and amino groups are responsible for the binding of the metal ions, and reduction of Cr(Ⅵ) to Cr(Ⅲ) takes place after Cr(Ⅵ) adsorbs electrostatically onto the surface of the biosorbents.
Key words:
biosorption; Synechococcus sp.; Cr(Ⅵ); Pb(Ⅱ); adsorption mechanism; adsorption kinetics;
1 Introduction
Water pollution of toxic heavy metal ions discharged from the industries and inhabited areas is seriously harmful to the health of human beings and the eco-system. Among these heavy metals, lead and chromium may be the severely pollutant sources due to their wide applications in industries, which may produce wastewater containing Pb(Ⅱ) and Cr(Ⅵ) at the concentrations higher than the recommended doses of 0.015 and 0.05 mg/L, respectively[1].
The traditional wastewater treatment methods including sludge separation, membrane separation, ion-exchange etc were often expensive and impractical in treatment of the wastewaters with heavy metal ion concentrations lower than 100 mg/L. However, biosorp- tion can be cost-effective[2-4]. In recent years, various kinds of biosorbents for removal of heavy metals have been investigated, which, for example, include bacteria [5-7], fungi[8-10], and algae[11-14]. These researches show that algae biosorbents may be much effective, especially when they are in dead cells form.
Microalgae biosorbents seem to be more promising than macroalgae (seaweeds), because on one hand, the cultivation of microalgae is normally easier and has higher production yield, and on the other hand, microalgae cells have higher performance and efficiency in biosorption due to their micron size and thus higher specific biosorption area of the biosorbents. Blue-green algae (Cyanobacteria) including Dunaliella, Spirulina (Arthrospira), Nostoc, Anabaena and Synechococcus were the typical examples that showed potential as biosorbents for efficient removal of heavy metals from wastewaters[12-14].
Cells of Synechococcus sp., typically in 2-3 μm, possess enough viscous extracellular polysaccharides at the early stationary phase of growth while maintaining single or dicoccus, and they usually aggregate with each other at the later stationary phase and death phase, suggesting that the early stationary phase is the better period for harvesting the cells. Though GARDEA- TORRESDEY et a[15] have studied Synechococcus as biosorbents for removal of metal ions, no comparative study on biosorption mechanisms of Cr(Ⅵ) and Pb(Ⅱ) on free or immobilized cells of Synechococcus has been reported. In this work, the free and immobilized cells of Synechococcus sp. harvested at the early stage of stationary phase were used as the adsorbents and the adsorption properties, adsorption kinetics and mechanisms of Cr(Ⅵ) and Pb(Ⅱ) were comparatively studied.
2 Experimental
2.1 Culture and harvest of cells of Synechococcus sp.
Pure strain of Synechococcus sp. was obtained from the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China. It was cultured in the HGZ medium (NaNO3 496 mg/L, K2HPO4 39 mg/L, CaCl2? 2H2O 36 mg/L, MgSO4·7H2O 75 mg/L, Na2SiO3·9H2O 58 mg/L, PⅡ metals solution 3 mL/L, and soil extract 3 mL/L) in a home-made rectangular bioreactor composed of 5 sub-rectangular compartments (each size 5 cm×5 cm×70 cm) at 25 ℃, under continuous illumination (2 klx) and air-lift mixing through the outside-columns of compartments. The growth curve was monitored with spectrophotometer at 540 nm (UV3000, Shimadzu, Japan). The cells were harvested at the early stage of stationary phase, and filtrated via membranes with pore size of 0.45 μm. Then the pellet was washed several times with deionized distilled water, dried by lyophilization, and stored at room temperature.
2.2 Immobilization of Synechococcus sp.
The powdered cells of Synechococcus sp. were immobilized using Ca-alginate via entrapment. 1 g of dry powder of biomass was mixed with 50 mL of 6% (v/w) Na-alginate solution, and the mixture was then introduced into 0.5 mol/L of CaCl2 solution through a peristaltic pump. After filtration, washing and freeze-dry process, the immobilized Synechococcus cell biosorbents were stored at 4 ℃ until being used.
2.3 Measurement of Zeta potentials of free cells
Zeta potentials of the free cells of Synechococcus sp. in solutions with pH values of 2-12 were measured by a Zeta potential analyzer (Delsa 440SX, Coulter, USA) at 25 ℃.
2.4 Determination of Cr(Ⅵ) and Pb(Ⅱ) ion concen- trations of samples
The concentrations of Cr(Ⅵ) and Pb(Ⅱ) in samples were determined by spectrophotometry (UV-3000, Shimadzu) with 1, 5-diphenylcarbazide in acid solution at 540 nm[16], and double chromogenic reagents dithizone and 4-(2-pyridylazo) resorcinol in aqueous solution at 520 nm[17]. Both methods took the reagent- blanks as the references, respectively.
2.5 Biosorption experiment
Each biosorption experiment was carried out in 150 mL conical flasks containing 50 mL metal ion solutions and 45 mg dry biomass (free and immobilized, respectively). The flasks were agitated at 70 r/min for Cr(Ⅵ) and 150 r/min for Pb(Ⅱ) for 2 h, respectively. After biosorption for a preset time (5 min, 10 min, 15 min, 20 min, 25 min, 30 min, 1 h, 2 h and 3 h), the mixtures were centrifuged at 4 000 r/min for 25 min, and the metal ions in the supernatants were analyzed by spectrophotometer as described above.
The amount of metal ions (mg) adsorbed by per gram of free or immobilized cell biosorbents was calculated by
qe=0.001(ci-ce)V/m (1)
qt=0.001(ci-ct)V/m (2)
where qe and qt are the amounts of metal ions adsorbed onto the unit amount of the biosorbents (mg/g) at equilibrium and any biosorption time, respectively; ci, ce and ct are the concentrations of metal ions in the bulk solutions at the initial, equilibrium and any biosorption time, respectively; V is the volume of bulk solution; and m is the mass of biosorbent.
2.6 FT-IR measurements
FT-IR spectra for both free and immobilized cells of biosorbents before and after adsorption of Pb(Ⅱ) or Cr(Ⅵ) as well as for K2Cr2O7 were obtained by the KBr pellet method operated on FT-IR analyzer (Nexus 670, Nicolet, USA).
3 Results and discussion
3.1 Zeta potentials of free cells of Synechococcus sp.
The Zeta potential curve of cells of Synechococcus sp. in different solutions (pH 2-12) is given in Fig.1. It is shown that free cells of Synechococcus sp. have an isoelectric point at about pH 3.0, and the cells surface shows electro-negative when pH>3. On the contrary, when pH<3 it shows electro-positive. This is in accordance with other kinds of algal cells[12, 18-19]. It could be expected that the acidic value of isoelectric point is due to the reciprocity among the numerous acidic units of the cell surface, such as, uronic acidic, carboxylic groups, and few alkaline units —NH2 and NH3+ groups, suggesting that at pH>3 the cell surface prefers to the electrostatic adsorption of positive metal ions because of the dissociation of acidic units; while at pH<3 it benefits the adsorption of negative metal ions for the protonation of alkaline units while numerous acidic units sustain un-dissociated.
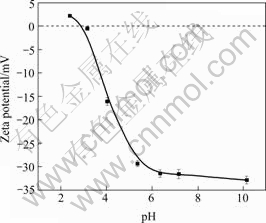
Fig.1 Zeta potential curve of free cells of Synechococcus sp.
3.2 Effects of pH on biosorption of Pb(Ⅱ) and Cr(Ⅵ)
Effects of pH on the biosorption of Pb(Ⅱ) or Cr(Ⅵ) are given in Fig.2. It is shown that an apparent transit in adsorption of both Pb(Ⅱ) and Cr(Ⅵ) on the free cells of biosorbent occurs at pH 3, the same point as isoelectric point discussed above, suggesting an electrostatic biosorption of the metal ions onto the cell surface of the biosorbents. Fig.2 also shows that the transit in adsorption of both Pb(Ⅱ) and Cr(Ⅵ) is shifted from pH 3.0 to 2.0 and Pb(Ⅱ) or Cr(Ⅵ) adsorption is decreased by immobilized cells, which may be due to the fact that the immobilization of cells by alginate provides more carboxylic groups that make the surface ionic charge more acidic, but the increase of granular size of the cells after immobilization leads to the reduction of the adsorption capacity. It is also noted from Fig.2 that the adsorption of Pb(Ⅱ) or Cr(Ⅵ) on Ca-alginate is much weaker than that of free or immobilized cell biosorbent,
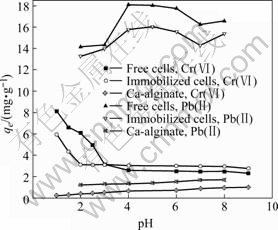
Fig.2 Effects of pH value on adsorptions of Cr(Ⅵ) and Pb(Ⅱ) ions (30 ℃, [Cr(Ⅵ)]ini=10 mg/L, [Pb(Ⅱ)]ini=20 mg/L, mass of adsorbents 45 mg)
biosorbent, suggesting that immobilization of cells by alginate does not significantly modify the adsorption properties of the cells. Immobilized cell biosorbent, however, shows better performance in actual multiple operation of adsorption.
It has been proposed that the major functional groups on the algal cell surface responsible for metal ions chelating are carboxylic groups, which have pKa value of 3.5-5.0. Besides carboxylic groups, the extracellular layer of Synechococcus that may be of glycoprotein provides other ions or proton side chains of the amino acids and sugar units, which will affect the surface charge density and the adsorption of the metal ions. Increase in pH may result in more dissociation of acidic groups, which leads to more electro-negatively charged ligands on the cell surface and favors the adsorption of Pb2+ ions. Optimal pH for removal of Pb(Ⅱ) ions in solution by algal biomass is reported at pH 5.0-6.0[20], at which Pb(Ⅱ) is in form of Pb2+ ions. Cr(Ⅵ) ions in the acidic solution are probably in diverse species [HCrO4]-, [Cr2O7]2-, [Cr4O13]2- and [Cr3O10]2- that are prone to adsorb on the protonated active sites of the biosorbent primarily[9]. By taking into account of the pH stability and isoelectric point of the biosorbents, pH 1-2 and pH 4-6 may be preferable to removing Cr(Ⅵ) and Pb(Ⅱ) by cells of Synechococcus.
3.3 Effects of temperature on biosorption of Pb(Ⅱ) and Cr(Ⅵ)
Effects of temperature on the biosorption of Pb(Ⅱ) and Cr(Ⅵ) are given in Fig.3. It is shown that the effects of temperature on adsorption of Pb(Ⅱ) and Cr(Ⅵ) on either free or immobilized cell biosorbents seem much weak, for the cases of immobilized form in the test range of 15-50 ℃.
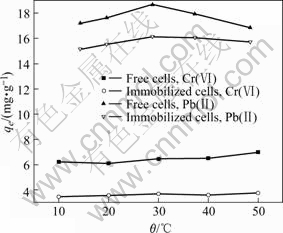
Fig.3 Effect of temperature on adsorptions of Cr(Ⅵ) and Pb(Ⅱ) ions ([Cr(Ⅵ)]ini=10 mg/L, [Pb(Ⅱ)]ini=20 mg/L, mass of adsorbents 45 mg, initial pH value 4.5 for Pb(Ⅱ) and 1.5 for Cr(Ⅵ), respectively)
3.4 Adsorption characteristics and effects of initial concentrations of Pb(Ⅱ) and Cr(Ⅵ)
The biosorption curves of Pb(Ⅱ) and Cr(Ⅵ) on free or immobilized cells at the preferable pH and ambient temperature are shown in Fig.4. It can be seen that the adsorption of Pb(Ⅱ) or Cr(Ⅵ) is very fast in the very early stage of biosorption, for instance, in 5 min, the biosorption quantities reach more than halves of the saturation values, and then it goes into a relatively slow stage until the saturation adsorption values of Pb(Ⅱ) and Cr(Ⅵ) are obtained at about 50 and 120 min, respectively. The relatively slow stage for each case can be approximately described by first order kinetic model. As shown in Fig.5, there is a linear relationship between ln ct and adsorption time t, which suggests that this stage may depend on the diffusion rate of the ions to the surface or through skeleton of the biosorbent, so topography and surface properties of the biosorbent may be the major influencing factors. The much longer time to reach the equilibrium for Cr(Ⅵ) than that for Pb(Ⅱ)suggests a possible difference in adsorption kinetic models between Cr(Ⅵ) and Pb(Ⅱ), where a plateau occurs only for adsorption of Pb(Ⅱ) (Fig.4).
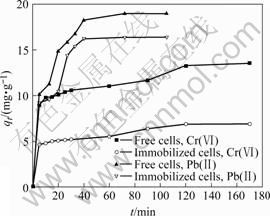
Fig.4 Adsorption curves of Cr(Ⅵ) and Pb(Ⅱ) ions (30 ℃, [Cr(Ⅵ)]ini=10 mg/L, [Pb(Ⅱ)]ini=20 mg/L, mass of adsorbents 45 mg, initial pH value 4.5 for Pb(Ⅱ) and 1.5 for Cr(Ⅵ), respectively)

Fig.5 Modulation of relatively slow stage of adsorption curves of Pb(Ⅱ) and Cr(Ⅵ) ions in Fig.4 by first order kinetics model
3.5 Modeling of adsorptions of Pb(Ⅱ) and Cr(Ⅵ)
In order to model the adsorptions of Pb(Ⅱ) and Cr(Ⅵ), Langmuir and Freundlich models[19] are adopted. The Langmuir equation is valid for monolayer reversible adsorption onto a surface with a finite number of identical sites and the model can be described by the Monod type equation:
![]() (3)
(3)
or its linear form:
![]() (4)
(4)
where ce and qe represent the metal ion concentration in the bulk phase and the amount of metal ions adsorbed on the adsorbent at adsorption equilibrium, respectively; qm is the maximal adsorption capacity; and kd is the Langmuir constant of the system at adsorption equilibrium.
When there are interactions among adsorbed molecules, a molecule attached to a surface may make it more or less difficult for another molecule to become attached to a neighboring site and this would lead to a deviation from the Langmuir adsorption equation. In this case, Freundlich model may be suitable, which can be expressed by
![]() (5)
(5)
or the linear equation in form of logarithm:
![]() (6)
(6)
where KF and n are the Freundlich constants. The Freundlich model is more general but provides no information on the monolayer adsorption capacity, in contrast to the Langmuir model.
After modeling the adsorption of Cr(Ⅵ) and Pb(Ⅱ) ions on either free or immobilized cell biosorbents by Langmuir and Freundlich models, respectively, the related Langmuir constants (qm and kd) and Freundlich constants (KF and n) along with the correlated coefficients (r 2) are listed in Table 1. Based on the r2 values, it can be seen that the adsorption of Pb(Ⅱ) can be described more suitably by Langmuir model, though Freundlich model is also descriptive, and the adsorption of Cr(Ⅵ) only obeys Freundlich model, indicating much probability of chemico-adsorption between Cr(Ⅵ) and biosorbents.
qm and kd derived from the Langmuir isotherm define the total capacity and binding affinity of the biosorbents for Pb(Ⅱ) ions. From Table 1, qm values for removal of Pb(Ⅱ) are in the sequence of free cell biosorbent>immobilized cell biosorbent, and the order of kd values is just contrary to that of qm values, which may be due to a decrease in the apparent surface adsorptive sites after the immobilization of the free cells.
For the cases of Freundlich isotherm where KF represents the adsorption coefficient, and n is related to the effect of concentration of metal ions, the adsorption feature is defined by both KF and n values, and to some extent, the n value contributes more significantly. From Table 1, KF value for Pb(Ⅱ) ion adsorption is larger by about 100 times for the free cell biosorbent and 10 times for the immobilized cell biosorbent than that for Cr(Ⅵ) ions adsorption, indicating that Pb(Ⅱ) ions have higher adsorption coefficients than Cr(Ⅵ) ions, and Pb(Ⅱ) ions have higher adsorption coefficients on the free cell biosorbent than on the immobilized cell biosorbent. Based on the values of n, the effect of concentration of Cr(Ⅵ) on Cr(Ⅵ) adsorption is about 450 times higher than that of Pb(Ⅱ) on Pb(Ⅱ) adsorption on the free cell biosorbent, and about 100 times higher than that of Pb(Ⅱ) on Pb(Ⅱ) adsorption on the immobilized cells biosorbent. It can be derived that the Pb(Ⅱ) adsorption is much affected by the apparent surface adsorptive sites of the biosorbents, and Cr(Ⅵ) adsorption is much affected by the initial concentration of metal ions that may principally affect the diffusion of ions onto the surface of biosorbents.
Table 1 Regression constants of adsorption isotherm models for adsorption of Pb(Ⅱ) and Cr(Ⅵ) ions from aqueous solutions on either free cells or immobilized cell biosorbents

3.6 Adsorption mechanisms of Pb(Ⅱ) and Cr(Ⅵ)
The comparison of IR spectra among K2Cr2O7, free cells biosorbent, free and immobilized cell biosorbents adsorbed by Cr2O72- or Pb2+ is shown in Fig.6. The frequencies of the spectrum bands and their assignments are listed in Table 2. Based on Ref.[20], the following can be derived from Fig.6 and Table 2.
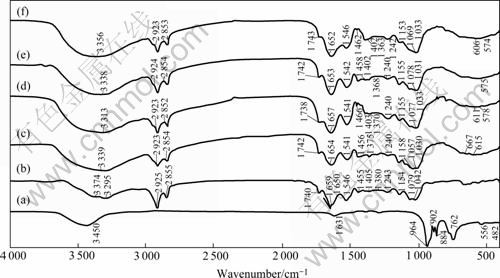
Fig.6 Comparison of FT-IR spectra among K2Cr2O7 (a), free cell (of Synechococcus sp.) biosorbent (b), free cell biosorbent adsorbed by Pb2+ (e) or Cr2O72- (c), immobilized cell biosorbent adsorbed by Pb2+ (f) or Cr2O72- (d)
Table 2 Frequencies and assignments of FTIR bands of free cells, Cr or Pb-bound free cells, Cr or Pb-bound immobilized cells
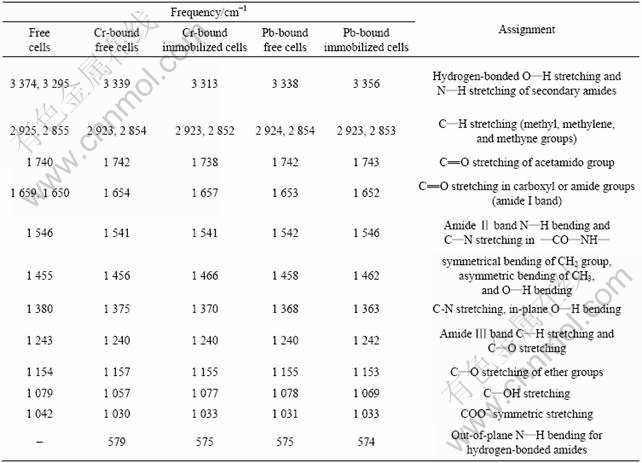
1) The larger broad bands in the region of 3 000- 3 500 cm-1 are assigned to the stretching of hydrogen-bonded O—H, N—H of secondary amides and NH3+. All of these are shifted and increased in intensity after biosorption of lead or chromium on both free and immobilized cell biosorbents, indicating the involvements of these groups in the biosorption of lead or chromium ions.
2) The stronger bands of 1 659, 1 546, 1 079 and 1 042 cm-1 in the spectra of free cell biosorbent, which are assigned to C=O stretching in carboxyl or amide groups (amide I band), amide Ⅱ band N—H bending and C—N stretching in —CO—NH—, C—OH stretching, and COO— symmetric stretching, respec- tively, are red-shifted and intensity-increased after biosorption of lead or chromium on either free or immobilized cell biosorbents, indicating that the carbonyl of carboxylic and amide groups, oxygenated forms and nitrogenated forms of carbon are involved in the biosorption of lead or chromium.
3) The larger bands in the region of 500-800 cm-1 that are assigned to out-of-plane N—H bending for hydrogen-bonded amides are apparently elongated and increased in intensity after biosorption of lead or chromium on both free and immobilized cell biosorbents, further indicating the involvement of amide nitrogen in the biosorption of lead or chromium.
4) The specific bands in the region of 700-980 cm-1 for K2Cr2O7 do not occur in Cr-bound free or immobilized cell, indicating that the reduction of Cr(Ⅵ) occurs during biosorption of chromium. By taking into account of the change in biosorption characteristic at the isoelectric point of the biosorbents (Figs.1 and 2), and different features in adsorption kinetics of Cr(Ⅵ) from Pb(Ⅱ) as discussed above, it may be deduced that the biosorption of Cr(Ⅵ) might follow two subsequent steps, that is, the adsorption of Cr2O72- by electrostatical force at the electro-positively active sites and the reduction of Cr2O72- to Cr3+ by the reductive groups on the surface of biosorbents. The first step is limited by the diffusion rate of Cr(Ⅵ) to the biosorbent surface and the diffusion rate is affected by the initial concentration of Cr(Ⅵ) in the bulk solution.
4 Conclusions
1) The adsorption processes of Cr(Ⅵ) and Pb(Ⅱ) on both free and immobilized cell biosorbents are apparently affected by pH and initial concentration of the metal ions in the bulk solution, but seem to be much weakly affected by temperature in the test range of 10-50 ℃.
2) The kinetic adsorption process consists of a very fast stage in the early several minutes, and a slower stage in the first order kinetic model.
3) The adsorption of Pb(Ⅱ) follows both Langmuir and Freundlich isotherm models, and that of Cr(Ⅵ) obeys only Freundlich model, in the test range of initial concentration of Cr(Ⅵ) and Pb(Ⅱ) of 0-700 mg/L.
4) FT-IR results indicate that carboxylic, alcoholic, amide and amino groups are responsible for the binding of the metal ions, and reduction of Cr(Ⅵ) to Cr(Ⅲ) takes place after Cr(Ⅵ) adsorbs electrostatically onto the surface of the biosorbents.
References
[1] VOLESKY B. Removal and recovery of heavy metals by biosorption [C]// VOLESKY B, ed. Biosorption of Heavy Metals. Boca Raton: CRC Press , 1990: 12-13.
[2] SHI Ai-hua, GAO Feng, YAN Wen-bin. Study on the processing technics of industry waste water with heavy metals [J]. Hunan Nonferrous Metals, 2007, 23(6): 41-43.
[3] GREGORIO CRINI. Non-conventional low-cost adsorbents for dye removal: A review [J]. Bioresource Technology, 2006, 97: 1061-1085.
[4] GAVRILESCU M. Removal of heavy metals from the environment by biosorption [J]. Engineering in Life Sciences, 2004, 4(3): 219-232.
[5] WANG Jian-long, CHEN Can. Biosorption of heavy metals by Saccharomyces cereviiae: A review [J]. Biotechnology Advances, 2006, 24(5): 427-451.
[6] IYER A, MODY K, JHA B. Biosorption of heavy metals by a marine bacterium [J]. Marine Pollution Bulletin, 2005, 50(3): 340-343.
[7] PINAKI S A, SUFIA K. KAZY, D′SOUZA S F. Radionuclide remediation using a bacterial biosorbent [J]. International Biodeterioration & Biodegradation, 2004, 54(2/3): 193-202.
[8] WANG J, MAO Z, ZHAO X. Response of Saccharomyces cerevisiae to chromium stress [J]. Process Biochem, 2004, 39: 1231-1235.
[9] ?ZER A, ?ZER D. Comparative study of the biosorption of Pb(Ⅱ), Ni(Ⅱ) and Cr(Ⅵ) ions onto S. cerevisiae: Determination of biosorption heats [J]. Hazard Mater, 2003, 100: 219-229.
[10] ATIMANAV GAUR, ALOK ADHOLEYA. Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils [J]. Current Science, 2004, 86(4): 528-534.
[11] DAVIS T A, VOLESKY B, MUCCI A. A review of the biochemistry of heavy metal biosorption by brown algae [J]. Water Res, 2003, 37: 4311-4330.
[12] D?NMEZ G, AKSU Z. Removal of chromium (Ⅵ) from saline wastewaters by Dunaliella species [J]. Process Biochem, 2002, 38: 751-762.
[13] CHOJNACKA K, CHOJNACKI A, G?RECKA H. Trace element removal by Spirulina sp. fom copper smelter and refinery effluents [J]. Hydrometallurgy, 2004, 73: 137-153.
[14] EL-SHEEKH M M, EL-SHOUNY W A, OSMAN M E H, EL- GAMMAL E W E. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents [J]. Environ Toxicol Pharmacol, 2005, 19: 357-365.
[15] GARDEA-TORRESDEY J L, ARENAS J L, WEBB R. Ability of immobilized Cyanobacteria to remove metal ions from solution and demonstration of metallothionin genes in various strains [J]. Hazard Substance Res, 1997, 3: 1-18.
[16] EATON A D, CLESCERI L S, GREENBERG A E. Standard methods for the examination of water and waste water, American Public Health Association (APHA) [J]. AWWA, WPCF: Washington, DC, 1995: 4-23.
[17] FAN X, DONG J. Spectrophotometric determination of Pb(Ⅱ) with double chromogenic reagents dithizone and 4-(2-pyridylazo) resorcinol in aqueous solution [J]. Phys Test Chem Anal Part B: Chem Anal, 2001, 37: 258-260.
[18] D?NMEZ G ?, AKSU Z, ?ZT?RK A, KUTSAL T. A comparative study on heavy metal biosorption characteristics of some algae [J]. Process Biochem, 1999, 34: 885-892.
[19] JALALI R, GHAFOURIAN H, ASEF Y, DAVARPANAHA S J, SEPEHRB S. Removal and recovery of lead using nonliving biomass of marine algae [J]. Hazard Mater, 2002, 92: 253-262.
[20] SHENG P X, TING Y P, CHEN J P, HONG L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms [J]. Colloid Interface Sci, 2004, 275: 131-141.
Foundation item: Project(50621063) supported by the National Natural Science Foudation of China
Corresponding author: SHEN Li; Tel: +86-731-8836943; E-mail: xiaolis@yahoo.com


