
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 1533-1539
Grain refining technique of AM60B magnesium alloy by MgCO3
CHEN Ti-jun, WANG Rui-quan, HUANG Hai-jun, MA Ying, HAO Yuan
Key Laboratory of Gansu Advanced Nonferrous Metal Materials, Lanzhou University of Technology, Lanzhou 730050, China
Received 27 May 2011; accepted 3 February 2012
Abstract:
The effects of grain refining parameters on microstructure of AM60B magnesium alloy with MgCO3 were investigated and then a refining technique was developed. Simultaneously, the corresponding mechanisms were discussed. The results indicate that increasing addition temperature of MgCO3 or pouring temperature is beneficial for obtaining fine grains. There is an optimal addition amount of 1.2% at the addition temperature of 790 ℃. Prolonging holding time at 790 ℃ will increase grain size. The grain refining technique that 1.2% MgCO3 is added at 790 ℃ followed by holding for 10 min and pouring can decrease the grain size from 348 μm of the un-refined alloy to 69 μm. The nucleation substrates are actually the Al4C3 particles formed from reactions between the MgCO3 and alloying elements in the melt. Besides the heterogeneous nucleation regime, growth restriction of the Al4C3 particles agglomerated at growing front is the other mechanism.
Key words:
AM60B magnesium alloy; grain refinement; MgCO3;
1 Introduction
Magnesium alloys, as the lightest metallic structural materials, are receiving increased attention in the fields of automobile and aircraft industries due to their low density and high specific strength. However, for the most commonly-used magnesium alloys, such as AM60, AZ91D and AZ31, their mechanical properties are relatively low and cannot meet the requirement of many applications [1]. It is well known that grain refinement can improve mechanical properties of most of alloys. Thus, a fine grain size is very important for overcoming this shortcoming of these alloys.
Unfortunately, there is no a commercially suitable grain refining technique for the Al-bearing magnesium alloys although several approaches have been developed. These approaches mainly include four categories, superheating [2], carbon inoculation [3], the Elfinal process [4] and grain refinement by other additives, such as Ca [5], Nb [6], Sr [5], B [7], Ti-B [8], B-C [9], Mn [10], Sc-Zr [11] and ZnO [12]. Comparatively, the carbon inoculation has good refining role and good adaptability to alloys’ compositions [13-17]. These reported additives include, but are not limited to, graphite, paraffin wax, lampblack, organic compound (C2Cl6 and C6Cl6), carbonates (CaCO3 and MgCO3), carbides (SiC, Al4C3 and CaC2, by bubbling the melt with CO, CO2 and CH4 gases). Among these additives, MgCO3 has specific advantages, such as low cost due to its abundant resources, low decomposition temperature and high carbon absorptivity due to its slow release speed of CO2 gas (compared with the organic compounds and bubbling method). Therefore, MgCO3 should be a suitable refiner in future commercial use.
However, there is no a reference to detailedly report the grain refining technique of magnesium alloys by MgCO3. The effects of refining parameters, such as addition amount, addition temperature, holding time and pouring temperature, on microstructure are unclear and the optimum parameters are unknown. In addition, most of the existing investigations about the carbon inoculation suggest that Al4C3 or Al2CO particles formed from reactions between the introduced C element and molten alloys may be the nucleation substrates of α-Mg. But there is no direct evidence to demonstrate it.
Therefore, in the present work, the effects of the refining parameters mentioned above on grain size of AM60B magnesium alloy are investigated and a refining technique is proposed. Simultaneously, the corresponding refining mechanisms are also discussed.
2 Experimental
The material used in this work was a commercial AM60B alloy and its composition is Mg-5.98Al- 0.343Mn-0.023Si (mass fraction, %). A quantity of AM60B alloys was processed in the sequence of remelting, adding MgCO3, holding, cooling to pouring temperature and pouring, and some cast rods with dimensions of d16 mm×150 mm then were obtained. The used mould was a permanent mould and its temperature was room temperature when pouring. During melting, the melt was protected by a RJ2 covering reagent. The detailed parameters are listed in Table 1, which indicates that four parameters, such as addition amount, addition temperature, holding time and pouring temperature, were considered, and when one parameter varied, the others were kept constants.
Small specimens with dimensions of d16 mm× 10 mm were cut from the obtained cast rods and finished and polished by standard metallographic techniques. Then they were etched by an aqueous solution containing glycerol, nitric acid, hydrochloric acid and acetic acid and observed on an optical microscope (OM). To delineate grain boundaries and quantitatively examine grain size, the specimens then were solutionized at 420 ℃ for 8 h and water-quenched. They were processed again according to the above procedures for preparing metallographic specimen and also observed under the OM. The obtained images were analyzed by Image-Pro Plus 5.0 software. Three images with magnification of 200 times for each specimen were examined and the average was taken as the specimen’s grain size. To verify grain refining mechanism, the specimen refined by 1.2% MgCO3 was observed and analyzed by scanning electron microscope (SEM), energy dispersive spectroscope (EDS) and electron microprobe analyzer (EPMA).
3 Results and discussion
3.1 Effects of grain refining parameters on grain size
3.1.1 Effect of addition amount
Figure 1 presents the typical microstructures of the AM60B alloys refined with different amounts of MgCO3. It shows that the un-refined alloy (refined by 0% MgCO3) has very developed dendrites and some of the dendrite arms are longer than 400 μm (Fig. 1(a)). After adding 0.3% MgCO3, the primary dendrites have been significantly refined (Fig. 1(b)) and the size continuously decreases as the addition amount increases (compared Figs. 1(b) and (c)). When the amount increases to 1.2%, a microstructure with uniform and fine equiaxed dendrites is obtained (Fig. 1(c). But as the amount further increases, the dendrites instead become more and more developed (compared with Fig. 1(d)). These changes can be more clearly seen from the solutionized microstructures and quantitative examination result shown in Figs. 2 and 3 respectively. It is found that the grain size is decreased from 348 μm to 69 μm after 1.2% MgCO3 was added (Fig. 3), which indicates that MgCO3 is an effective grain refiner for AM60B alloy.
Table 1 Used grain refining parameters of AM60B magnesium alloy by MgCO3
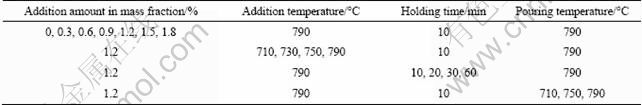
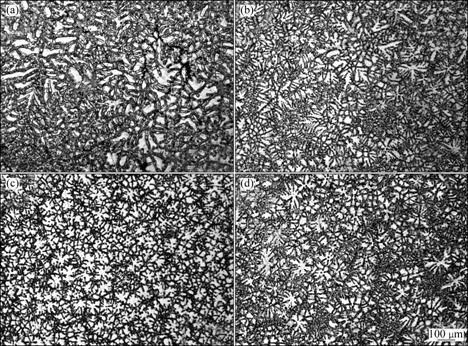
Fig. 1 Microstructures of AM60B alloys refined by different amounts of MgCO3: (a) 0%MgCO3; (b) 0.3%MgCO3; (c) 1.2%MgCO3; (d) 1.5%MgCO3
The most accepted standpoint about carbon inoculation is the heterogeneous nucleation taking Al4C3 particles as substrates, and the Al4C3 particles form from reaction of the introduced C and Al in the melt [13-17]. It can be expected that the substrate number should increase as the addition amount increases. Figure 3 shows that only adding 0.3% MgCO3 can lead the grain size to decrease from 348 μm to 197 μm, which means that MgCO3 has a relatively high grain refining potency. When the addition increases to 1.2%, the present result implies that the number of the formed effective substrates reaches a saturated level that the melt can contain under the present conditions. When the number exceeds this level, the frequency of mutual collision, agglomeration and coalescence may sharply increases. Furthermore, the density of the substrates (Al4C3) is higher than that of the melt, so the coalescence also accelerates their settlement, that is to say the extra MgCO3 may result in the decrease of the effective substrate number. Based on these standpoints, the grain size changes in such tendency with the addition amount can be easily understood.
3.1.2 Effect of addition temperature
Figure 4 presents the variation of grain size with addition temperature of MgCO3. It shows that the grain size continuously decreases as the temperature rises. It is known that MgCO3 will decompose into CO2 and MgO. The C element that is needed by forming the Al4C3 substrates should be from reactions between the released CO2 and alloying elements in the melt. The higher the temperature is, the more easily the decomposition is. So, it can be expected that proper increasing the temperature can increase the substrate number. In addition, in view of thermodynamics, the substrate size should decrease with increasing the temperature when the formed substrate amount is given, and thus their number must increase. More importantly, the decrease in size can also improve their nucleation potency [18]. Because of these three reasons, the grain size continuously decreases with increasing the addition temperature.
Furthermore, there should be other nucleation substrates such as Al-Fe or Al-Mn-Fe intermetallics, magnesium or aluminum oxides, or similar nonmetallic inclusions in the melt besides the Al4C3 particles [19,20]. Some of them with relatively low melting point may partially melt at higher temperature. So, one large-sized substrate may separate into two or more small-sized ones. This not only increases the substrate number, but also increases their nucleation potency due to the decrease of their size [12]. Namely, the superheating mechanism will play an important role at higher temperatures [18,19]. This may be the fourth reason why the grain size changes in such manner. Finally, it should be noted that magnesium alloys are easily to be oxidized at high temperature, and thus this temperature should be decreased as low as possible according to the actual requirement for grain size.
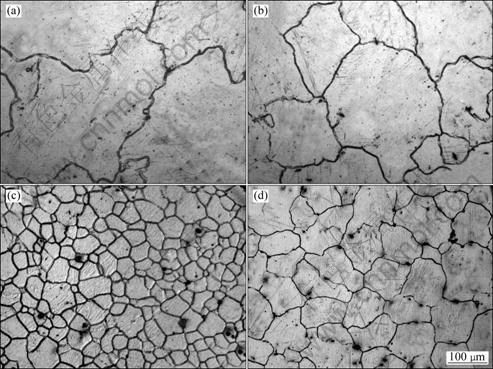
Fig. 2 Microstructures of AM60B alloys shown in Fig. 1 after being solutionized at 420 ℃ for 8 h: (a) 0%MgCO3; (b) 0.3%MgCO3; (c) 1.2%MgCO3; (d) 1.5%MgCO3
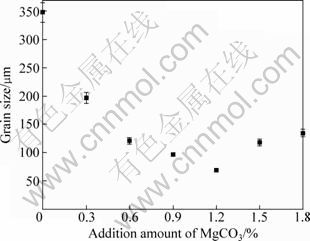
Fig. 3 Variation of grain size with addition amount of MgCO3
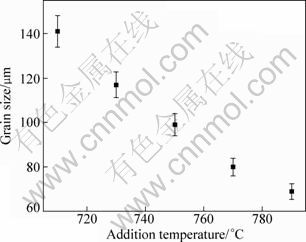
Fig. 4 Variation of grain size with addition temperature
3.1.3 Effect of holding time
Figure 5 gives the variation of grain size with holding time at 790 ℃, which shows that the grain size continuously increases as the time is prolonged. It is well known that inoculation fading is a common phenomenon during grain refining treatment. In the present work, because of the higher density of Al4C3 substrates than the melt, it can be proposed that the fading mainly should ascribe to the agglomeration and settlement of the substrates. Figure 5 indicates that the grain size increases from 69 μm to 126 μm during the holding period of 10-60 min and the increase rate basically keeps at a constant of 1 μm/min during the whole period. Although the average increase rate is not too high, there is no a stage during that the rate is relatively low. So, the melt treated by MgCO3 should be poured as soon as possible.
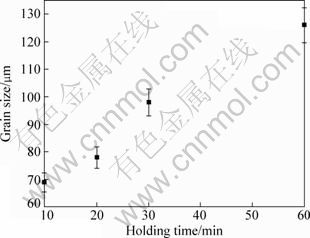
Fig. 5 Variation of grain size with holding time at 790 ℃
3.1.4 Effect of pouring temperature
Figure 6 gives the variation of grain size with pouring temperature. It shows that the grain size decreases as the temperature rises. The highest pouring temperature is 790 ℃, similar to the addition temperature or holding temperature. Under this condition, the melt is treated by MgCO3 at 790 ℃, followed by holding for 10 min and pouring at the same temperature. But under the other conditions, the treated melts must be cooled to pouring temperatures because the addition temperature is higher than the pouring temperature. The lower the pouring temperature, the longer the time needed by cooling, and thus the more serious the inoculation fading. In addition, it should be pointed out that the growth or coalescence of the substrates can be enhanced as the melt’s temperature reduces, not only decreasing the substrate number, but also reducing the nucleation potency. Namely, the inoculation fading under these conditions also includes the coalescence of substrates besides the agglomeration and settlement discussed above. Furthermore, similar to the effect of addition temperature, the role played by superheating regime should also be decreased. Due to these two reasons, the grain size decreases with increasing pouring temperature.
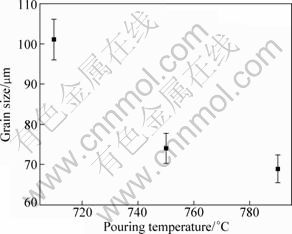
Fig. 6 Variation of grain size with pouring temperature
3.2 Grain refining mechanism
As described in the section of introduction, the most accepted standpoint about the carbon inoculation is the heterogeneous nucleation taking Al4C3 or Al2CO particles as substrates [13,14]. For the inoculation by SiC particles, it is proposed that the SiC particles themselves can also serve as nucleation substrates besides the formed Al4C3 particles. Furthermore, JIN et al [20] have suggested that the introduced C element diffuses out from the growing α-Mg grains and then generates constitutional undercooling at the growing front, which does not only prevent the grains from growth, but also accelerates the formation of new nuclei. But this viewpoint is disproved by QIAN and CAO [16] and they considered that the grain refinement is attributed to Al4C3 particles. However, there are still no direct proofs to demonstrate which kind of particles (Al4C3 or Al2CO) is the nucleation substrate and what the specific details are.
In the present work, as shown by the arrow in Fig. 7(a), there is always a small particle in the center of each equiaxed dendrite of the alloy refined with 1.2% MgCO3. This particle can be considered the nucleus of the grain. The result from EDS indicates that this particle consists of C, Al, O and Mg (Fig. 7(b)). It is believed that the peak of Mg element should originate from the magnesium matrix [17]. So, the particle actually includes C, Al and O.
It is can be expected that the added MgCO3 will decompose into MgO and CO2 because the melt temperature of 790 ℃ (the addition or holding temperature) is obviously higher than its decomposition temperature of 420 ℃ [21]. The decomposition reaction can be expressed as:
MgCO3=CO2+MgO,ΔG=117458-169.708T(298-1000 K)(1)
It was reported that CO2 gas can react with Mg element to form C element at above 400 ℃ [22]. So, the released CO2 gas then reacts with Mg element in the melts and C is reduced according to the reaction:
CO2+2Mg=C+2MgO (2)
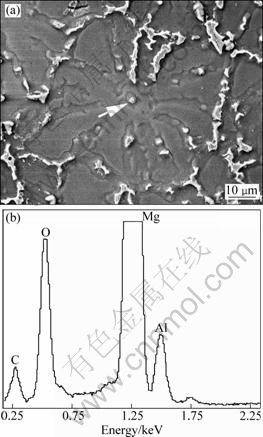
Fig. 7 SEM micrograph (a) and EDS result (b) about particle marked by arrow in (a)
Finally, the reduced C element reacts with Al element to form Al4C3 through the transformation [23]:
3C+4Al=Al4C3, ΔG=203 kJ/mol (3)
So, it can be concluded that, based on the theoretical analysis, the final C-containing product is Al4C3, but not a compound that consists of the three elements of C, Al and O. Furthermore, considering from thermodynamics, it is also impossible to form Al-C-O compounds, such as Al2OC particles that can act as nucleation substrates of α-Mg grains in view of crystal mismatch, due to low oxygen potential or small Al2OC activity in the melt [16,20]. It is known that Al4C3 is extremely reactive to water, and thus the Al4C3 particles can react with water during polishing of the specimens through the reaction [3,15]:
Al4C3+12H2O=4Al(OH)3+3CH4 (4)
which leads the Al4C3 to become into an Al-C-O compound. So, the O element in the particle results from the reaction (4) and the particle only includes two elements of Al and C, namely, it is Al4C3.
Figure 8 implies that the Al4C3 particles not only exist in the center of dendrites as nucleation substrates, but also distribute in the eutectic structures between the dendrites. It can be supposed that the Al4C3 phase is the first precipitation phase during solidification. Some of them act as the nucleation substrates of primary grains and finally situate in the center of dendrites. The others are pushed by growing front and agglomerate at the solid/liquid interfaces to restrict grain growth. Finally, they agglomerate in the lastly solidified regions, and the eutectic structures between dendrites. Therefore, it can be concluded that the grain refinement of MgCO3 has two mechanisms, heterogeneous nucleation taking the Al4C3 particles as substrates and growth restriction of the agglomerated Al4C3 particles at growing front.
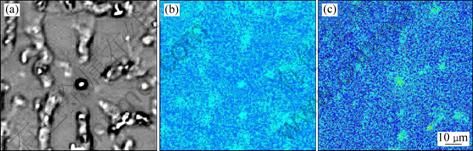
Fig. 8 Back scattered electron micrograph (a), Al (b) and C (c) maps of AM60B alloy refined by 1.2% MgCO3
4 Conclusions
1) MgCO3 is an effective grain refiner for AM60B magnesium alloy. Increasing addition temperature or pouring temperature is beneficial for obtaining fine grains. There is an optimal addition amount of 1.2% at the addition temperature of 790 ℃. Prolonging the holding time at 790 ℃ can increase grain size. A grain refining technique was developed as follows: 1.2% MgCO3 is added into the melt at 790 ℃, the melt then is held for 10 min and poured. The grain size can be decreased from 348 μm for the un-refined alloy to 69 μm.
2) The nucleation substrates are actually the Al4C3 particles formed from the reactions between the MgCO3 and alloying elements in the melt. Besides the heterogeneous nucleation regime, growth restriction of the agglomerated Al4C3 particles at the growing front is the other mechanism.
References
[1] Eliezer D, Aghion E, Froes F H. Magnesium science, technology and applications [J]. Adv Perform Mater, 1998, 5: 201-212.
[2] Cao P, Qian M, StJohn D H. Mechanism for grain refinement of magnesium alloys by superheating [J]. Scripta Mater, 2007, 56: 633-636.
[3] Motegi T. Grain-refining mechanisms of superheat-treatment of and carbon addition to Mg-Al-Zn alloys [J]. Mater Sci Eng A, 2005, 413-414: 408-411.
[4] Dahle A K, Lee Y C, Nave M D, Schaer P L, StJohn D H. Development of the as-cast microstructure in magnesium-aluminium alloys [J]. J Light Met, 2001, 1: 61-72.
[5] Hirai K, Somekawa H, Takigawa Y, Higashi K. Effect of Ca and Sr addition on mechanical properties of a cast AZ91D magnesium alloy at room and elevated temperature [J]. Mater Sci Eng A, 2005, 403: 276-280.
[6] Zhang J H, Tang D X, Meng J. Effect of Nd on the microstructure, mechanical properties and corrosion behavior of die-cast Mg-4Al-based alloy [J]. J Alloys Compd, 2008, 464: 556-564.
[7] Suresh M, Srinivasan A, Ravi K R, Pillai U T S, Pai B C. Influence of boron addition on the grain refinement and mechanical properties of AZ91 Mg alloy [J]. Mater Sci Eng A, 2009, 525: 207-210.
[8] Wang Y, Zeng X, Ding W. Effect of Al-4Ti-5B master alloy on the grain refinement of AZ31 magnesium alloy [J]. Scripta Mater, 2006, 54: 269-273.
[9] Ma G, Han G, Liu X. Grain refining efficiency of a new Al-1B-0.6C master alloy on AZ63 magnesium alloy [J]. J Alloys Compd, 2010, 491: 165-169.
[10] Cao P, Qian M, StJohn D H. Effect of manganese on grain refinement of Mg-Al based alloys [J]. Scripta Mater, 2006, 54: 1853-1858.
[11] Wang S C, Chou C P. Effect of adding Sc and Zr on grain refinement and ductility of AZ31 magnesium alloy [J]. J Mater Proc Technol, 2008, 197: 116-121.
[12] Fu H M, Qiu D, Zhang M X, Wang H, Kelly P M, Taylor J A. The development of a new grain refiner for magnesium alloys using the edge-to-edge model [J]. J Alloys Compd, 2008, 456: 390-396.
[13] Gunther R, Hartig C, Bormann R. Grain refinement of AZ31 by (SiC)P: Theoretical calculation and experiment [J]. Acta Mater, 2006, 54: 5591-5597.
[14] Easton M A, Schiffl A, Yao J Y, Kaufmann H. Grain refinement of Mg-Al(-Mn) alloys by SiC additions [J]. Scripta Mater, 2006, 55: 379-382.
[15] Lu L, Dahle A K, StJohn D H. Grain refinement efficiency and mechanism of aluminium carbide in Mg–Al alloys [J]. Scripta Mater, 2005, 53: 517-522.
[16] Qian M, Cao P. Discussions on grain refinement of magnesium alloys by carbon inoculation [J]. Scripta Mater, 2005, 52: 7415-7419.
[17] ZHANG M X, KELLEY P M, QIAN M, TAYLOR J A. Crystallography of grain refinement in Mg-Al alloys [J]. Acta Materialia, 2005, 53: 3261-3270.
[18] StJohn D H, Qian M, Easton M A, Cao P, HILDEBRAND Z. Grain refinement of magnesium alloys [J]. Metall Mater Trans A, 2005, 36: 165-169
[19] Vinotha D, Raghukandan K, Pillai U T S, Pai B C. Grain refining mechanisms in magnesium alloys—An overview [J]. Trans Ind Inst Met, 2009, 62:521-532.
[20] Jin Q L, Eom J P, Lim S G, Park W W, You B S. Reply to comments on “grain refining mechanism of a carbon addition method in an Mg-Al magnesium alloy” [J]. Scripta Mater, 2005, 52: 421-423.
[21] Pei H B, Xu B Q, Li Y F. Study on the thermal decomposition behavior of magnesite in carbothermic reduction extraction process for magnesium in vacuum [J]. Light Met, 2010(1): 46-50.
[22] Shih T S, Chung C B, Chong K Z. Combustion of AZ61A under different gases [J]. Mater Chem Phy, 2002, 74: 66-73.
[23] Enrique R R, Paul F B, Edgar L C. Influence of carbon on the interfacial contact angle between alumina and liquid aluminum [J]. Sur Interf Analy, 2003, 35: 151-155.
AM60B镁合金的MgCO3晶粒细化技术
陈体军,王瑞权,黄海军,马 颖,郝 远
兰州理工大学 甘肃省有色金属新材料重点实验室,兰州 730050
摘 要:研究以MgCO3作为AM60B镁合金晶粒细化剂的细化工艺参数对微观组织的影响,从而开发一种细化工艺,同时,讨论其相应的细化机理。结果表明,升高MgCO3的添加温度或升高浇铸温度有利于获得细晶组织;在790 ℃时添加,最佳的加入量为1.2%,延长在此温度的保温时间使晶粒尺寸增大;在790 ℃时加入1.2% MgCO3,随后保温10 min浇铸,可使晶粒尺寸从未细化的348 μm减小到69 μm;MgCO3与熔体中的合金元素反应所形成的Al4C3颗粒是异质形核的基底;除异质形核机理外,聚集在生长前沿的Al4C3颗粒对晶体生长的阻碍是晶粒细化的另一原因。
关键词:AM60B镁合金;晶粒细化;MgCO3
(Edited by YANG Hua)
Foundation item: Project (G2010CB635106) supported by the National Basic Research Program of China; Project (NCET-10-0023) supported by the Program for New Century Excellent Talents in University of China
Corresponding author: CHEN Ti-jun; Tel: +86-931-2976573; E-mail: chentj@lut.cn, chentj1971@126.com
DOI: 10.1016/S1003-6326(11)61352-6
Abstract: The effects of grain refining parameters on microstructure of AM60B magnesium alloy with MgCO3 were investigated and then a refining technique was developed. Simultaneously, the corresponding mechanisms were discussed. The results indicate that increasing addition temperature of MgCO3 or pouring temperature is beneficial for obtaining fine grains. There is an optimal addition amount of 1.2% at the addition temperature of 790 ℃. Prolonging holding time at 790 ℃ will increase grain size. The grain refining technique that 1.2% MgCO3 is added at 790 ℃ followed by holding for 10 min and pouring can decrease the grain size from 348 μm of the un-refined alloy to 69 μm. The nucleation substrates are actually the Al4C3 particles formed from reactions between the MgCO3 and alloying elements in the melt. Besides the heterogeneous nucleation regime, growth restriction of the Al4C3 particles agglomerated at growing front is the other mechanism.


