
Synthesis and electrochemical performance of 5V spinel LiNi0.5Mn1.5O4 prepared by solid-state reaction
SUN Qiang(孙 强), LI Xin-hai(李新海),WANG Zhi-xing(王志兴), JI Yong(季 勇)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 24 March 2008; accepted 3 June 2008
Abstract:
Spinel compound LiNi0.5Mn1.5O4 with high capacity and high rate capability was synthesized by solid-state reaction. At first, MnCl2·4H2O and NiCl2·6H2O were reacted with (NH4)2C2O4·H2O to produce a precursor via a low-temperature solid-state route, then the precursor was reacted with Li2CO3 to synthesize LiNi0.5Mn1.5O4. The effects of calcination temperature and time on the physical properties and electrochemical performance of the products were investigated. Samples were characterized by thermal gravimetric analysis(TGA), scanning electron microscopy(SEM), X-ray diffractometry(XRD), charge-discharge tests and cyclic voltammetry measurements. Scanning electron microscopy(SEM) image shows that as calcination temperature and time increase, the crystallinity of the samples is improved, and their grain sizes are obviously increased. It is found that LiNi0.5Mn1.5O4 calcined at 800 ℃ for 6 h exhibits a typical cubic spinel structure with a space group of Fd3m. Electrochemical tests demonstrate that the sample obtained possesses high capacity and excellent rate capability. When being discharged at a rate as high as 5C after 30 cycles, the as-prepared LiNi0.5Mn1.5O4 powders can still deliver a capacity of 101 mA?h/g, which shows to be a potential cathode material for high power batteries.
Key words:
lithium ion battery; LiNi0.5Mn1.5O4; cathode; solid-state reaction;
1 Introduction
High energy and high power rechargeable Li-ion cells are key components of the portable, entertainment, computing and hybrid electric vehicles[1-2]. One approach of increasing the power density of lithium ion batteries is to enhance its operation voltage by utilizing high-voltage cathodes. Transition metal-substituted spinel materials (LiMxMn2-xO4, M=Cr, Co, Fe, Ni, Cu) showed high voltage plateau at around 5V[3-7], and hence have been widely studied for advanced lithium ion batteries. The capacity and voltage plateau in Li/LiMxMn2-xO4 cells strongly depend on the kind of transition metals(M) and their contents. Among those materials, LiNi0.5Mn1.5O4 received great attention for its dominant potential plateau at around 4.7 V. Moreover, LiNi0.5Mn1.5O4 showed the highest discharge capacity (146.7 mA?h/g) with stable cycleability at this high potential.
In recent years, many methods to synthesize LiNi0.5Mn1.5O4 have been reported, including solid-state method[8], sol-gel method[9], co-precipitation method [10], composite carbonate process[11], molten salt method[12], emulsion drying method[13], and ultrasonic spray pyrolysis method[14]. Different synthesizing methods can result in products with different properties[15]. In synthesized LiNi0.5Mn1.5O4, secondary phases such as NiO and LixNi1-xO usually exist in the products, which is due to the oxygen loss at high temperature and can deteriorate electrochemical behaviors[16]. Fortunately, the oxygen loss occurring at high temperatures can be mostly recovered by low rate cooling in low temperature annealing[17-18]. In this work, an improved preparation of LiNi0.5Mn1.5O4 by solid reaction in air is described, and the electrochemical properties of resulting powders are studied.
2 Experimental
Appropriate amounts of chemicals, NiCl2?6H2O and MnCl2?4H2O, were thoroughly mixed (cationic ratio of Ni to Mn of 1?3). Subsequently, 20% (mass fraction) excess of (NH4)2C2O4?H2O was added to the mixture, then the mixture was ground for about 0.5 h to ensure complete reaction. The obtained mixture was dried in air at 120 ℃ for 10 h and then was calcined at 400 ℃ for 3 h in air to form a precursor containing Ni and Mn (Ni-Mn for short). In order to investigate the effects of calcination temperature and calcination time on the physical properties and electrochemical performance of the products, the precursor was calcined at 750-900 ℃ for different time after being mixed with stoichiometric Li2CO3, then kept at 600 ℃ for 24 h in air to obtain products.
Thermogravimetric(TG) analysis was performed on a thermogravimetric analyzer (Universal V4.0C TA Instruments: SDT Q600 V8.0 Build 95) at a heating rate of 10 ℃/min in a constant flow of extra dry air at 100 mL/min. The powder X-ray diffraction (XRD, Rint- 2000, Rigaku) measurement using Cu Kα radiation was employed to identify the crystalline phase of the synthesized materials. The particle size and morphology of LiNi0.5Mn1.5O4 powders were observed by scanning electron microscope (SEM, JEOL, JSM-5600LV) with an accelerating voltage of 20 kV.
The electrochemical properties of the products were tested in cells with metallic lithium as anode electrode. For cathode fabrication, the prepared powders were mixed with 10% acetylene black and 10% polyvinylidene fluoride in N-methyl pyrrolidinone(NMP) until a slurry was obtained. And then, the blended slurries were pasted onto an aluminum current collector, and the electrode was dried at 120 ℃ for 10 h in vacuum. The test cell consisted of the cathode and lithium foil anode separated by a porous polypropylene film, and the electrolyte solution was 1 mol/L LiPF6 in a mixture of ethylene carbonate(EC), etheyl methyl carbonate(EMC) and dimethyl carbonate(DMC) in a volume ratio of 1?1?1. The assembly of the cells was carried out in a dry Ar-filled glove box. The cells were charged and discharged over a voltage range of 3.5-4.9 V versus Li/Li+ electrode at room temperature. Cyclic voltammogram was measured at a sweep rate of 0.1 mV/s between 3.5 and 5.2 V.
3 Results and discussion
In the preparation of Ni-Mn precursor via a low-temperature solid-state route, the following reaction could take place[19]:
NiCl2?6H2O+3MnCl2?4H2O+4(NH4)2C2O4?H2O→
NiC2O4?2H2O+3MnC2O4?2H2O+8NH4Cl+14H2O (1)
Since the reaction was performed at room temperature, the mixture obtained may contain plenty of nanosized oxalate compounds. It is well known that oxalate compounds often have low decomposition temperature, thus it is highly possible to synthesize LiNi0.5Mn1.5O4 by simply calcining the precursor at low temperatures.
Thermogravimetry(TG) analysis tests for oxalate compound of Ni-Mn and the mixture of Ni-Mn precursor and Li2CO3 were carried out, respectively. The TG curve for oxalate compound is shown in Fig.1(a). The mass loss terminates at about 400 ℃, and two discrete mass losses are observed due to removal of water at 25-280 ℃ and the decomposition of oxalate compound of Ni-Mn at 280-400 ℃. When the temperature goes up beyond 400 ℃, just small mass losses are observed, which indicates that a properly stable Ni-Mn oxide is obtained. In order to save energy, based on the TG data, a calcining temperature of 400 ℃ is used to prepare the Ni-Mn precursor. After being mixed with stoichiometric Li2CO3, the Ni-Mn precursor shows three mass losses, as indicated in Fig.1(b). Mass losses below 600 ℃ are associated with the water release and pyrolysis of the Li2CO3, while no mass loss is observed between 600 ℃ and 780 ℃, which indicates that the water and CO2 have been released completely below 600 ℃. However, when the temperature increases to 780 ℃, a third mass loss occurs, which is likely caused by oxygen loss. The oxygen content in the ambient atmosphere is expected to play an important role in the stability of the cubic spinel. It is proved that the strategy of slow cooling or annealing treatment at low temperature is very necessary to optimize the oxygen content of LiNi0.5Mn1.5O4 because the oxygen loss of LiNi0.5Mn1.5O4 occurs at high tem- perature[17]. Therefore, annealing at 600 ℃ for 20 h is introduced in order to compensate for oxygen loss.
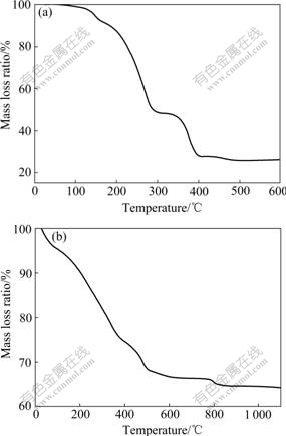
Fig.1 TG curves for oxalate compound of Ni-Mn (a) and Ni-Mn precursor mixed with Li2CO3 (b)
Fig.2 shows the XRD patterns of LiNi0.5Mn1.5O4 powders obtained after calcining at different temperatures for 6 h. All samples are confirmed as a typical cubic spinel structure with a space group of Fd3m. As the calcination temperature increases from 750 to 900 ℃, the diffraction peaks become sharper and stronger, indicating that the crystallinity is improved. It is noted that as the calcination temperature increases to 850 ℃, the slight peaks representing NiO impurities appear on the left of the (222), (400) and (440) peaks in the XRD pattern, as shown in Fig.2.
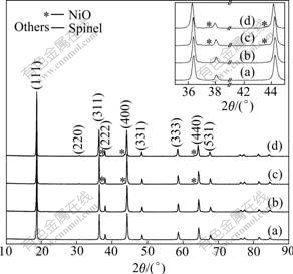
Fig.2 XRD patterns of LiNi0.5Mn1.5O4 powders calcined at different temperatures for 6 h: (a) 750 ℃; (b) 800 ℃; (c) 850℃; (d) 900 ℃
The morphologies of LiNi0.5Mn1.5O4 powders obtained at various temperatures were observed using a SEM, and the results are shown in Fig.3. It can be seen that 750 ℃-calcined sample displays undeveloped crystallization and a little agglomeration. As calcination temperature increases, the grain size grows obviously, and 900℃-calcined sample has the maximum particle size (approximate 2.5 μm). This implies a sharp decline in the specific surface area of the samples as calcination temperature is elevated. It is also found that the samples calcined at the temperatures higher than 800 ℃ represent clear polyhedral shape, indicating well- developed crystallinity. These are in the agreement with the results from XRD.
The charge and discharge curves of LiNi0.5Mn1.5O4 powders prepared at different temperatures are shown in Fig.4, which are carried out at current density of 0.2C (C represents 1 Li+ ion exchanged in 1 h equivalent to 148 mA/g). It can be seen that its discharge capacity increases with the calcination temperature increasing under 800 ℃. This may originate from relatively higher crystallinity of 800 ℃-calcined sample and less electrolyte decomposition products formed on the surfaces of this sample. When the temperature increases beyond 850 ℃, the discharge capacity shows a little decline. This is closely related to the high calcination temperature employed (>800 ℃) which may result in the presence of undesired impurities such as NiO or LixNi1-xO in the final product, and these impurities may, to a great extent, deteriorate the electrochemical performance of LiNi0.5Mn1.5O4. It is noted that the discharge curves of 850 ℃- and 900 ℃-calcined samples still present shorter plateaus in the potential region from 3.9C to 4.2C besides the higher plateaus of around 4.7 V, which results from the transfer reaction between Mn3+ and Mn4+. It can also be seen from Fig.4 that the samples calcined at relatively higher temperatures (800-900 ℃) have much larger initial discharge capacities (>130 mA?h/g) than those calcined at lower temperatures (750 ℃, 125 mA?h/g), although the lower discharge plateaus occur due to the existence of Mn3+ ions.
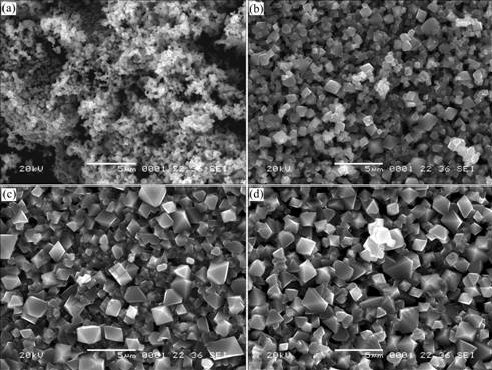
Fig.3 SEM images of LiNi0.5Mn1.5O4 products obtained by calcining at different temperatures for 6 h: (a) 750 ℃; (b) 800 ℃; (c) 850 ℃; (d) 900 ℃
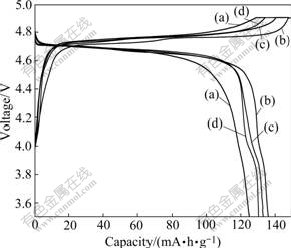
Fig.4 Charge and discharge curves of LiNi0.5Mn1.5O4 prepared by calcining at different temperatures for 6 h: (a) 750 ℃; (b) 800 ℃; (c) 850 ℃; (d) 900 ℃
The XRD patterns of LiNi0.5Mn1.5O4 powders calcined at 800 ℃ for different time are shown in Fig.5. It is seen that all diffraction peaks of three samples are ascribed to a cubic spinel structure with a space group of Fd3m. As the calcination time increases, the diffraction peaks become sharper and stronger. When products are calcined for 12 h, some diffraction peaks of impurities are observed, which indicates that there are some impurities, such as NiO or LixNi1-xO. Fig.6 shows the SEM images of as-prepared samples. It can be seen that the particles grow up from 0.5, 1 to 2.5 μm with increasing the calcination time from 1, 6 to 12 h. When samples are calcined for more time, such as 6 h and 12 h, the clearer octahedral shape characteristic of cubic spinel is observed, indicating the well-developed crystallinity.
The charge-discharge curves of as-prepared LiNi0.5Mn1.5O4 cycled between 3.5 V and 4.9 V at the rate of 0.2C are shown in Fig.7. It is seen that the calcination time has a significant effect on the charge-discharge profiles of LiNi0.5Mn1.5O4. When being calcined at 800 ℃ for 1 h, LiNi0.5Mn1.5O4 shows only one plateau at around 4.7 V, which demonstrates that most of lost oxygen is already recovered by annealing process. When the calcination time is more than 6 h, LiNi0.5Mn1.5O4 materials exhibit two plateaus at around 4.7 V and 4 V, which are attributed to the Ni2+/Ni4+ and Mn3+/Mn4+ redox couples, respectively. Moreover, as the calcination time reaches 12 h, there is a clearer discharging plateau at around 4 V than that of 6 h. This indicates that even though annealing process is employed for 12 h, the products could not compensate for the oxygen loss. In addition, the discharge capacity increases from 128 to 136 mA?h/g with increasing the calcination time from 1 to 6 h. This observation can be understood in terms of the optimized crystallinity in the final product after calcining for a relative long time. 6 h- and 12 h-calcined LiNi0.5Mn1.5O4 samples show almost equal capacity when being discharged at the rate of 0.2C, but the capacity of 12 h-calcined LiNi0.5Mn1.5O4 (133 mA?h/g) is slightly smaller than that of 6 h-calcined sample. This behavior is most likely associated with the increased amount of Mn3+ and NiO impurities which caused by oxygen deficiency after long calcination treatment.

Fig.5 XRD patterns of LiNi0.5Mn1.5O4 powders obtained by calcining at 800 ℃ for different time: (a) 1 h; (b) 6 h; (c) 12 h
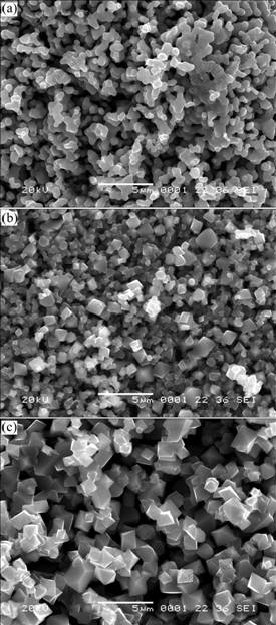
Fig.6 SEM images of LiNi0.5Mn1.5O4 powders obtained by calcining at 800 ℃ for different time: (a) 1 h; (b) 6 h; (c) 12 h
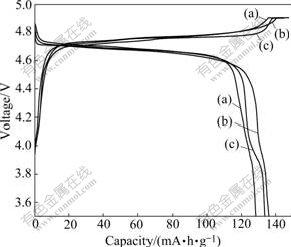
Fig.7 Charge and discharge curves of LiNi0.5Mn1.5O4 powders calcined at 800 ℃ for different time: (a) 1 h; (b) 6 h; (c) 12 h
In Fig.8 the cyclic voltammetry(CV) curves of LiNi0.5Mn1.5O4 samples prepared under optimization conditions (calcining at 800 ℃ for 6 h) are plotted in the potential range from 3.5 V to 5.2 V at a scanning rate of 0.1 mV/s. The major doublet redox peaks at around 4.5-5 V originate from the Ni2+/Ni4+ redox couple and the ordering of lithium on 8a sites at 50% filling[16]. Whereas the small doublet redox peaks in the potential region from 3.9 to 4.2 V are ascribed to the Mn3+/Mn4+ redox, which results from the existence of small amount of NiO or LixNi1-xO impurities in the sample. With the increase of the charge-discharge cycles, the major doublet redox peaks at around 4.5-5V of the sample gradually overlap, and become a little broad peak after 30 cycles. At the same time, the potential difference between the oxidation and reduction peaks gets smaller, and the reduction-peak currents slightly increase. This indicates that the electrochemical reversibility and activity of the product increase with the increase of charge-discharge cycle, and the corresponding electrode polarization decreases. The results of CV experiments are in good agreement with those of the preceding charge-discharge experiments.
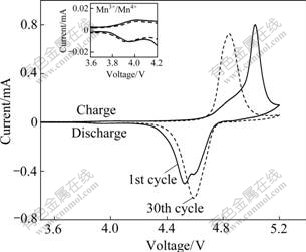
Fig.8 Cyclic voltammetry curves of LiNi0.5Mn1.5O4 sample obtained by calcining at 800 ℃ for 6 h (Scanning rate 0.1 mV/s, potential 3.5 -5.2 V)
It is well-known that rate capability plays an important role in lithium ion batteries. Here, high-rate electrochemical performance of LiNi0.5Mn1.5O4 prepared under optimization conditions (calcining at 800 ℃ for 6 h) was evaluated. Fig.9 shows cycling performance of LiNi0.5Mn1.5O4 cycled between 3.5 and 4.9 V, charged at the rate of 0.1C and discharged at the 0.2C, 1C and 5C for 30 times, respectively. Before comparison of high- rate test, the cell was charge-discharged at a rate of 0.1C in a voltage range of 3.5-4.9 V for two times. It can be seen that both the plateau voltage and the discharge capacity decrease with the current density increasing. The product shows a considerable stable cycling behavior and the cycling retention rate after 30 cycles is over 96% at low current density of 0.2C. When the current density increases to 1C and 5C, although the samples exhibit an inferior cycling behavior, the cycling retention rates of products after 30 cycles can still reach 90% and 81%, respectively. Therefore, it can be concluded that as-prepared products possess excellent electrochemical performance even at high rate.
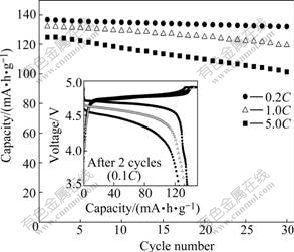
Fig.9 Cycle performances of LiNi0.5Mn1.5O4 powders calcined at 800 ℃ for 6 h
4 Conclusions
1) LiNi0.5Mn1.5O4 products can be classified as the typical cubic spinel Fd3m structure with simple phase. Sample characterizations indicate that the crystallinity and morphology of the products can be improved by increasing the calcination temperature and time.
2) The electrochemical performance measurements demonstrate that LiNi0.5Mn1.5O4 products calcined at 800℃ for 6 h have much improved capacity and excellent rate capability. The discharge capacity reaches up to 136 mA?h/g at a current density of 0.2C, while it retains as high as 101 mA?h/g even at a high current density of 5C after 30 cycles.
References
[1] YOSHIO N. Lithium ion secondary batteries: Past 10 years and the future [J]. Journal of Power Sources, 2001, 100: 101-106.
[2] LIU Li-ying, TIAN Yan-wen, ZHAI Yu-chun, XU Cha-qing. Influence of Y3+ doping on structure and electrochemical performance of layered Li1.05V3O8 [J]. Trans Nonferrous Met Soc China, 2007, 17(1): 110-115.
[3] MOLENDA J, MARZEC J, SWIERCZEK K, OJCZUK W, ZIEMNICKI M, MOLENDA M, DROZDEK M, DZIEMBAU R. The effect of 3d substitutions in the manganese sublattice on the charge transport mechanism and electrochemical properties of manganese spinel [J]. Solid State Ionics, 2004, 171: 215-227.
[4] OHZUKU T, TAKEDA S, IWANAGA M. Solid-state redox potentials for Li[Me1/2Mn3/2]O4 (Me: 3d-transition metal) having spinel-framework structures: A series of 5 volt materials for advanced lithium-ion batteries [J]. Journal of Power Sources, 1999, 81/82: 90-94.
[5] OHZUKU T, ARIYOSHI K, TAKEDA S, SAKAI Y. Synthesis and characterization of 5V insertion material of Li[FeyMn2-y]O4 for lithium-ion batteries [J]. Electrochimica Acta, 2001, 46: 2327-2336.
[6] BONINO F, PANERO S, SATOLLI D, SCROSATI B. Synthesis and characterization of Li2MxMn4-xO8 (M=Co, Fe) as positive active materials for lithium-ion cells [J]. Journal of Power Sources, 2001, 97/98: 389-392.
[7] TERADA Y, YASAKA K, NISHIKAWA F, KONISHI T, YOSHIO M, NAKAI I. In situ XAFS analysis of Li(Mn, M)2O4 (M=Cr, Co, Ni) 5V cathode materials for lithium-ion secondary batteries [J]. Journal of Solid State Chemistry, 2001, 156: 286-291.
[8] FANG Hai-sheng, WANG Zhi-xing, LI Xin-hai, GUO Hua-jun, PENG Wen-jie. Exploration of high capacity LiNi0.5Mn1.5O4 synthesized by solid-state reaction [J]. Journal of Power Sources, 2006, 153: 174-176.
[9] ANDOUNI N, ZAGHIB K, GENDRON F, MAUGER A, JULIEN C M. Magnetic properties of LiNi0.5Mn1.5O4 spinels prepared by wet chemical methods [J]. Journal of Magnetism and Magnetic Materials, 2007, 309: 100-105.
[10] FAN Yu-kai, WANG Jian-ming, YE Xue-bo, ZHANG Jian-qing. Physical properties and electrochemical performance of LiNi0.5Mn1.5O4 cathode material prepared by a coprecipitation method [J]. Materials Chemistry and Physics, 2007, 103: 19–23.
[11] LIU G Q, WANG Y J, QI L, LI W, CHEN H. Synthesis and electrochemical performance of LiNi0.5Mn1.5O4 spinel compound [J]. Electrochimica Acta, 2005, 50: 1965–1968.
[12] KIM J H, MYUNG S T, SUN Y K. Molten salt synthesis of LiNi0.5Mn1.5O4 spinel for 5V class cathode material of Li-ion secondary battery [J]. Electrochimica Acta, 2004, 49: 219–227.
[13] MYUNG S T, KOMABA S, KUMAGAI N, YASHIRO H, CHUNG H T, CHO T H. Nano-crystalline LiNi0.5Mn1.5O4 synthesized by emulsion drying method [J]. Electrochimica Acta, 2002, 47: 2543–2549.
[14] PARK S H, SUN Y K. Synthesis and electrochemical properties of 5V spinel LiNi0.5Mn1.5O4 cathode materials prepared by ultrasonic spray pyrolysis method [J]. Electrochimica Acta, 2004, 50: 431–434.
[15] ARREBOLA J C, CABALLERO A, CRUZ M. Crystallinity control of a nanostructured LiNi0.5Mn1.5O4 spinel via polymer-assisted synthesis: A method for improving its rate capability and performance in 5V lithium batteries [J]. Adv Funct Mater, 2006, 16: 1904-1912.
[16] ALCANTARA R, JARABA M, LAVELA P, TIRADA J L. Optimizing preparation conditions for 5V electrode performance, and structural changes in Li1-xNi0.5Mn1.5O4 spinel [J]. Electrochimica Acta, 2002, 47: 1829–1835.
[17] ZHANG Bao, WANG Zhi-xing, GUO Hua-jun. Effect of annealing treatment on electrochemical property of LiNi0.5Mn1.5O4 spinel [J]. Trans Nonferrous Met Soc China, 2007, 17(2): 287-290.
[18] FANG Hai-sheng, WANG Zhi-xing, ZHANG Bao, LI Xin-hai, LI Guang-she. High performance LiNi0.5Mn1.5O4 cathode materials synthesized by a combinational annealing method [J]. Electrochemistry Communications, 2007, 9: 1077-1082.
[19] FANG Hai-sheng, LI Li-ping, LI Guang-she. A low-temperature reaction route to high rate and high capacity LiNi0.5Mn1.5O4 [J]. Journal of Power Sources, 2007, 167: 223-227.
Foundation item: Project(2007CB613607) supported by the National Basic Research Program of China
Corresponding author: WANG Zhi-xing, Tel: +86-731-8836633; E-mail: zxwang@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(08)60248-4
(Edited by YANG Bing)
Abstract: Spinel compound LiNi0.5Mn1.5O4 with high capacity and high rate capability was synthesized by solid-state reaction. At first, MnCl2·4H2O and NiCl2·6H2O were reacted with (NH4)2C2O4·H2O to produce a precursor via a low-temperature solid-state route, then the precursor was reacted with Li2CO3 to synthesize LiNi0.5Mn1.5O4. The effects of calcination temperature and time on the physical properties and electrochemical performance of the products were investigated. Samples were characterized by thermal gravimetric analysis(TGA), scanning electron microscopy(SEM), X-ray diffractometry(XRD), charge-discharge tests and cyclic voltammetry measurements. Scanning electron microscopy(SEM) image shows that as calcination temperature and time increase, the crystallinity of the samples is improved, and their grain sizes are obviously increased. It is found that LiNi0.5Mn1.5O4 calcined at 800 ℃ for 6 h exhibits a typical cubic spinel structure with a space group of Fd3m. Electrochemical tests demonstrate that the sample obtained possesses high capacity and excellent rate capability. When being discharged at a rate as high as 5C after 30 cycles, the as-prepared LiNi0.5Mn1.5O4 powders can still deliver a capacity of 101 mA?h/g, which shows to be a potential cathode material for high power batteries.


