Trans. Nonferrous Met. Soc. China 26(2016) 3052-3058
Isothermal section of Al-Ti-Zr ternary system at 1073 K
Kai-li  , Feng YANG, Zhi-yun XIE, Hua-shan LIU, Ge-mei CAI, Zhan-peng JIN
, Feng YANG, Zhi-yun XIE, Hua-shan LIU, Ge-mei CAI, Zhan-peng JIN
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 26 October 2015; accepted 11 April 2016
Abstract:
Through alloy sampling combined with diffusion triple technique, phase equilibria in Al-Ti-Zr ternary system at 1073 K were experimentally determined with electron probe microanalysis (EPMA). Experimental results show that there is a solid solution β(Ti,Zr) which dissolves Al up to 16.3% (mole fraction). Ti and Zr can substitute each other in most Ti-Al and Al-Zr binary intermediate phases to a certain degree while the maximum solubility of Zr in Ti3Al and TiAl reaches up to 17.9% and 4.0% (mole fraction), respectively. The isothermal section consists of 16 single-phased regions, 27 two-phased regions and 14 three-phased regions. No ternary phase was detected.
Key words:
Al-Ti-Zr ternary system; phase equilibrium; diffusion triple; solubility;
1 Introduction
Coupled with high temperature strength and oxidation resistance, low density and low thermal conductivity make Ti-Al alloys potential replacements to steels and superalloys applied in gas engines and turbines. Among the Ti-Al alloys, γ-TiAl and TiAl3 based intermetallics are important and widely applied alloys [1-6]. Additions of elements have been used to further improve the mechanical performance of titanium aluminium alloys [7-9]. For instance, the addition of Zr improves ductility and toughness, and thus improves processability of Ti-Al base alloys at room temperature [10-12]. Furthermore, the addition of Zr benefits the formation of the Al3Zr phase, which may serve as potential nucleation core for α(Al), thus results in a homogeneous microstructure with improved machinability of the related Ti-Al alloys [13,14]. To better understand the influence of alloying element Zr in Ti-Al alloys, phase equilibria of the related Al-Ti-Zr system are essential.
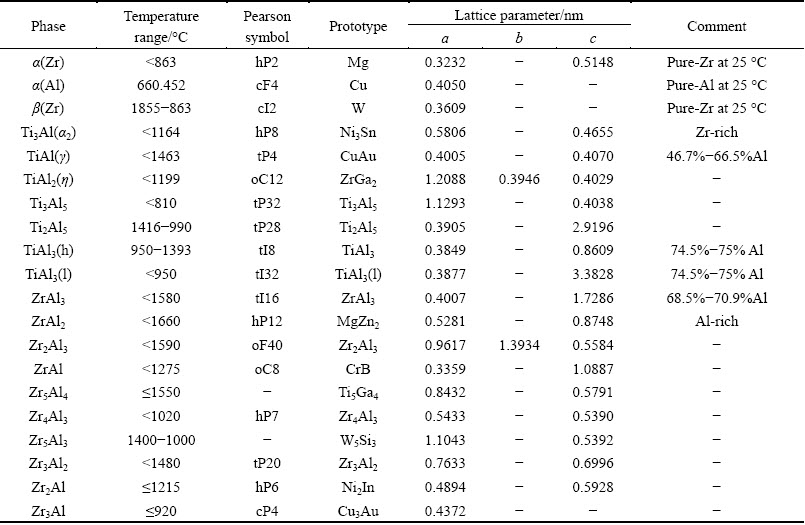
The constituent binary systems have been well experimetally studied and thermal dynamically assessed in the literatures. According to Ref. [15], the Ti-Zr binary system is an isomorphous system. The Al-Zr and Ti-Al binary systems were successfully assessed by WANG et al [16] and WITUSIEWICZ et al [17], respectively, with the intermediate compounds included, i.e., Zr3Al, Zr2Al, Zr5Al3, Zr3Al2, Zr4Al3, Zr5Al4, ZrAl, Zr2Al3, ZrAl2, ZrAl3 and TiAl3(h), TiAl3(l), Ti2Al5, TiAl2, Ti3Al5, TiAl, and Ti3Al. Information of the stable solid phases in these binary systems is summarized in Table 1.
Phase relations in the Al-Ti-Zr ternary system are far from being accomplished. Only an isothermal section of the Al-Ti-Zr system at 1273 K is available [18]. It is obvious that the phase diagram is the map of material design. In order to assist the design and fabrication of Ti-Al based alloys, extensive investigation of phase equilibria in the Al-Ti-Zr ternary system is necessary. The present work is to determine the isothermal sections of Al-Ti-Zr ternary system at 1073 K experimentally by using diffusion triple technique combined with alloy sampling.
2 Experimental
The present work was to experimentally study the phase relations of Al-Ti-Zr system at 1073 K by diffusion triple and alloy sampling. Ti (99.999%), Al (99.99%) and Zr (99.99%) (mass fraction) were used as starting materials, bought from China New Metal Materials Technology Co., Ltd.. Predetermined amount of each raw material was weighed with analytical balance, followed by arc-melted in a water-cooled copper crucible under argon atmosphere with titanium as getter material placed in the arc chamber. To ensure good homogeneity of the samples, all samples were turned over before each melting and re-melted at least 3 times. The mass losses did not exceed 1%.
The method to fabricate diffusion triple can be found in Ref. [20]. The Ti, Zr and deliberately prepared TiAl3 button ingot were machined into the proper shapes (cuboid with 3 mm × 3 mm × 10 mm and cylindrical shells with rectangular openings) through wire-electrode cutting. Surfaces of the metallic pieces were ground, polished, cleaned and then assembled into the geometry shown in Fig. 1. The assembled diffusion triple was then loaded into cans made of commercial purity Ti (schematically shown in Fig. 1(b)), and subjected to hot isostatic pressing (HIP) at 1073 K, 200 MPa for 4 h. The top and bottom caps of the HIP can were electron beam welded.
The obtained diffusion triple and ternary button alloys were sealed in a silica capsule back-filled with high purity argon, and then annealed at 1073 K for 2400 h in a diffusion furnace (temperature error is within ±5 °C). After annealing, the samples were taken out and quenched into water.
The annealed specimens were polished and microstructural investigations of the alloys were carried out using electron probe microanalyser (JXA-8800R, JEOL, Japan). Approach to determination of phase equilibria from diffusion triple relies on the assumption of local equilibrium at the phase interface, and details of the approach can also be found in Ref. [20]. The compositions of equilibrated alloys in this study were the average values of five measurements. Standard deviation of the measured concentration is ±0.6%. The total mass fractions of Al, Zr and Ti in each phase are in the range of 97%-103%, so the effect of reactions between the samples and silica capsules could be neglected.
Table 1 Stable solid phases in three binary systems [18,19]
3 Results and discussion
3.1 Analysis of diffusion triple
Figure 2 illustrates the backscatter electron images of the diffusion triple annealed at 1073 K for 2400 h. During the long-term diffusion treatment, extensive interdiffusion among Al, Ti and Zr took place, and many phases were formed. By performing EPMA analysis, extensive information about phase equilibria was obtained. It can be seen from Fig. 2(a) that several diffusion layers (corresponding to different phases) exist in the tri-junction. By measuring the composition of phases near triple points, 3 three-phased fields could be obtained, including γ(TiAl) + Zr(Al,Ti)2 + β(Ti,Zr), γ(TiAl) + α2(Ti3Al) + β(Ti,Zr), α2(Ti3Al) + α(Ti) + β(Ti,Zr) and 9 two-phase fields ε(TiAl3) + η(TiAl2), η(TiAl2)+ γ(TiAl), γ(TiAl) + α2(Ti3Al), α2(Ti3Al) + α(Ti), α(Ti) + β(Ti,Zr), α2(Ti3Al) + β(Ti,Zr), γ(TiAl) + β(Ti,Zr), Zr(Al,Ti)2 + β(Ti,Zr), γ(TiAl)+Zr(Al,Ti)2. Furthermore, from the TiAl3-Zr diffusion area shown in Fig. 2(b), 5 layers can be distinguished, i.e., α(Zr), (Zr,Ti)3Al, (Zr,Ti)2Al, (Zr,Ti)3Al2 and (Zr,Ti)4Al3. Consequently, 4 additional two-phased equilibria, α(Zr) + (Zr,Ti)3Al, (Zr,Ti)3Al + (Zr,Ti)2Al, (Zr,Ti)2Al + (Zr,Ti)3Al2 and (Zr,Ti)3Al2 + (Zr,Ti)4Al3, were obtained. The phase equilibria from the diffusion triple are summarized in Table 1.
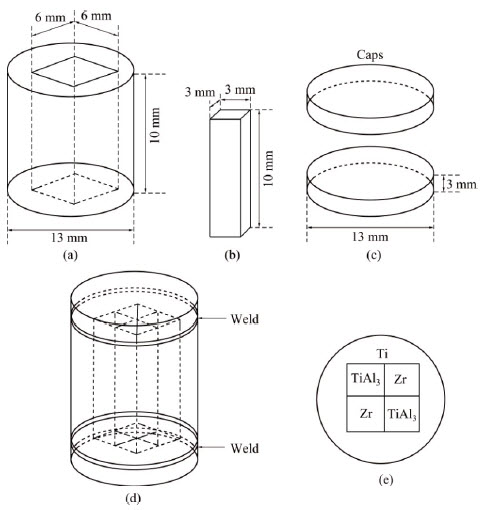
Fig. 1 Schematic illustration of components (a-c), assembly (d), and cross-sectional view (e) of TiAl3-Ti-Zr diffusion triple
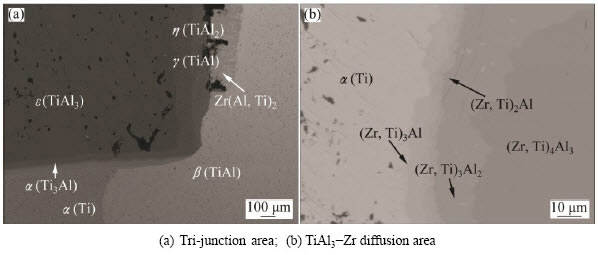
Fig. 2 Backscatter electron SEM image of TiAl3-Ti-Zr diffusion multiple annealed at 1073 K for 2400 h
3.2 Analysis of equilibrated alloys
Back-scattered electron (BSE) images of typical ternary Al–Ti–Zr alloys after annealing at 1073 K are presented in Fig. 3 and Fig. 4. All the samples display a well-defined three-phased or two-phased structure, implying that equilibrium has been reached or nearly reached for the annealed Al-Ti-Zr alloys. In the Al63Ti25Zr12 (mole fraction, %, here after) alloy, the three-phased microstructure Zr(Al,Ti)2+η(TiAl2)+γ(TiAl) was observed (Fig. 3(a)). As seen in Fig. 3(b), the microstructure of alloy Al50Ti30Zr20 consists of dark γ(TiAl) phase, and light gray Zr(Al,Ti)2 phase, indicating that this alloy is located in the two-phased field of γ(TiAl) + Zr(Al,Ti)2. BSE micrograph of the alloy Al60Ti5Zr35 is shown in Fig. 3(c), which is featured with a three-phased equilibrium of Zr(Al,Ti)2 + Zr2Al3 + (Zr,Ti)4Al3. Equilibrium between (Zr,Ti)3Al3 and Zr2Al3 was also observed in the alloys Al50Ti5Zr45 (see Fig. 3(d)).
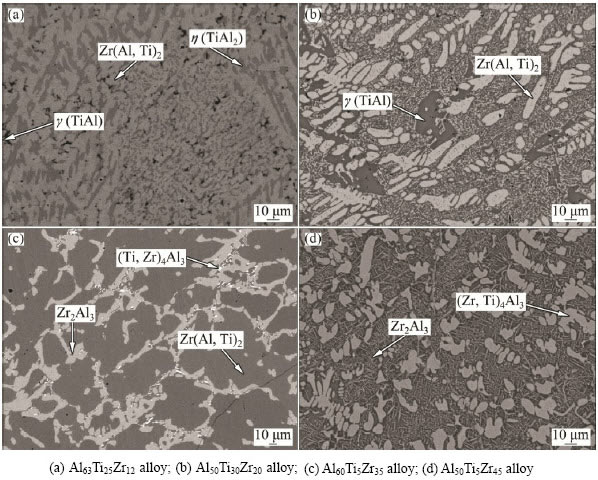
Fig. 3 BSE images of typical Al-Ti-Zr ternary alloys annealed at 1073 K for 2400 h
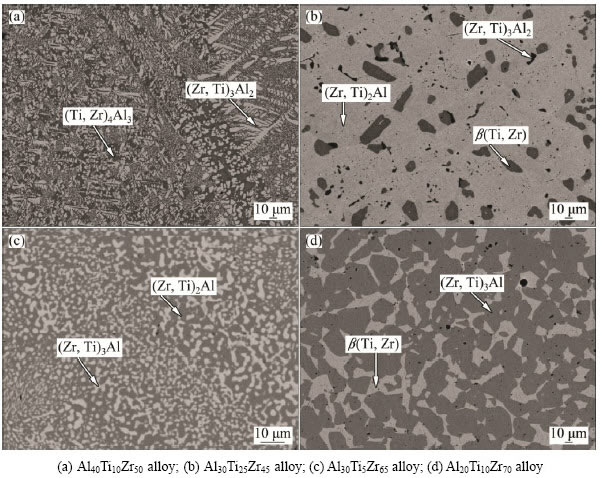
Fig. 4 BSE images of typical Al-Ti-Zr ternary alloys annealed at 1073 K for 2400 h
Figure 4(a) shows the microstructure of the alloy Al40Ti10Zr50, where the two-phased equilibrium (Zr,Ti)4Al3 + (Zr,Ti)3Al2 occurs. The alloy Al30Ti25Zr45 contained three phases (see Fig. 4(b)), i.e., the black (Zr,Ti)3Al2 phase, the dark gray β(Ti,Zr) phase and the light gray (Zr,Ti)2Al phase. As is shown in Fig. 4(c), (Zr,Ti)3Al and (Zr,Ti)2Al coexist in the Al30Ti5Zr65 alloy. In addition, from Fig. 4(d), it is seen that the alloy Al20Ti10Zr70 contains (Zr,Ti)3Al and β(Ti,Zr) in equilibrium.
3.3 Isothermal section of 1073 K
The measured compositions of all phases in equilibria in the Al-Ti-Zr ternary system at 1073 K are summarized in Table 2. Based on Tables 2 and 3, the isothermal section at 1073 K is constructed as presented in Fig. 5. In the isothermal section, 8 three-phased regions were completely determined, including ε(TiAl3) + (Zr,Ti)Al3 + η(TiAl2), η(TiAl2) + (Zr,Ti)Al3 + Zr(Al,Ti)2, γ(TiAl) + η(TiAl2) + Zr(Al,Ti)2, γ(TiAl) + Zr(Al,Ti)2 + β(Ti,Zr), γ(TiAl) + α2(Ti3Al) + β(Ti,Zr), α2(Ti3Al) + α(Ti) + β(Ti,Zr), Zr(Al,Ti)2 + Zr2Al3 + (Zr,Ti)4Al3 and (Zr,Ti)2Al + β(Ti,Zr) + (Zr,Ti)3Al2. Additionally, other 6 three-phased regions, i.e., L + ε(TiAl3) + (Zr,Ti)Al3, Zr(Al,Ti)2 + (Zr,Ti)4Al3 + β(Ti,Zr), (Zr,Ti)4Al3 + Zr2Al3 + ZrAl, (Zr,Ti)4Al3 + (Zr,Ti)3Al2 + β(Ti,Zr), (Zr,Ti)3Al + (Zr,Ti)2Al + β(Ti,Zr) and (Zr,Ti)3Al + β(Ti,Zr) + α(Zr) could be further deduced. What’s more, According to the Al-Zr binary phase diagram [16], phase ZrAl should be stable at 1073 K. So, it is reasonably deduced that the three-phased region, Zr2Al3 + ZrAl + (Zr, Ti)4Al3, exists at 1073 K.
By the way, in most Ti-Al and Zr-Al binary compounds, Zr atoms and Ti atoms can mutually substitute to a certain degree. The only exception is Zr(Al,Ti)2, whose homogeneity range extends along the isoconcentrate of Zr, meaning substitution of Al by Ti. Moreover, at 1073 K, the maximum solubilities of Ti in (Zr,Ti)Al3, (Zr,Ti)2Al, (Zr,Ti)4Al3, (Zr,Ti)3Al2 and (Zr,Ti)3Al can be up to 12.0%, 11.6%, 13.5%, 14.4% and 8.0% (mole fraction), respectively. Meanwhile, the maximum solubilities of Zr in TiAl3 and TiAl2 were 6.1% and 17.2%, respectively. As to the Zr(Al,Ti)2, the maximum solubility of Ti was 13.8%. Zr2Al3 and ZrAl do not show remarkable composition ranges at 1073 K.
Table 2 Equilibrium composition of Al-Ti-Zr ternary system obtained from diffusion couple in present work

Additionally, compared with the isothermal sections of the Al-Ti-Zr system at 1273 K [18], the obvious difference exists in the phase (Zr,Ti)5Al3, which disappears at 1073 K, resulting in the change of the phase relationship related to it. Moreover, with temperature decreasing, solubility of Zr in ε(TiAl3) phase decreases from 9.3% to 6.0%, and in γ(TiAl) from 9.0% to 4.0%. As for the BCC solid solution β(Ti, Zr), the maximum solubility of Al was 16.3% at 1073 K, lower than 25.5% at 1273 K [18].
Table 3 Measured compositions of phases in alloys annealed at 1073 K
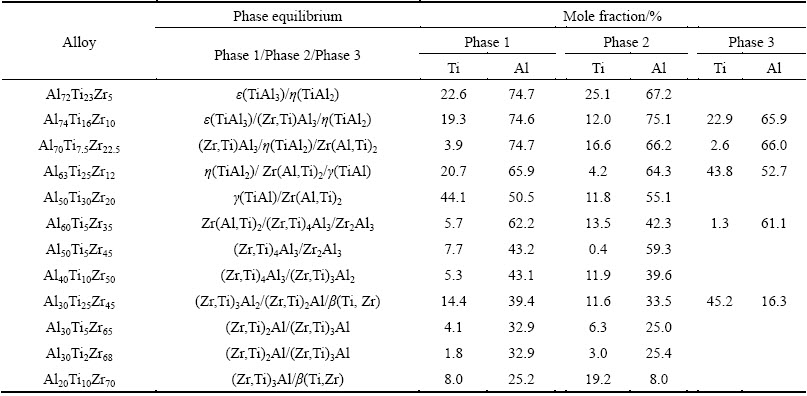
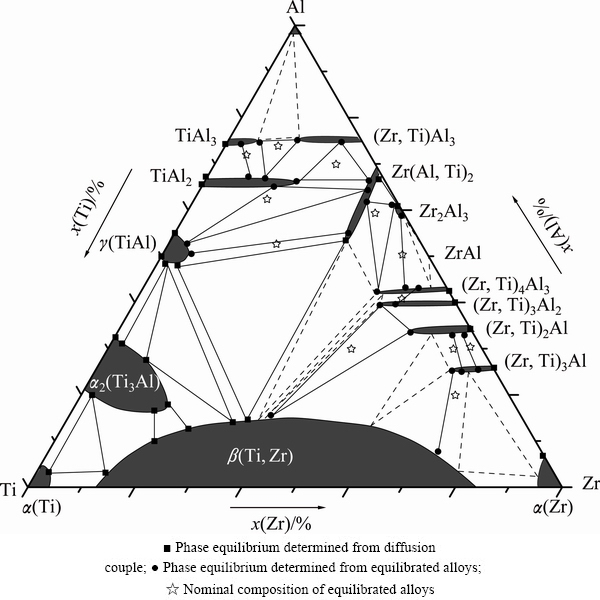
Fig. 5 Experimentally determined isothermal sections of Al-Ti-Zr system at 1073 K
4 Conclusions
1) The isothermal section of the Al-Ti-Zr ternary system at 1073 K was established by analyzing diffusion triple and equilibrated alloys through EPMA.
2) Ti and Zr can substitute each other in most Ti-Al and Al-Zr binary intermediate phases to a certain degree.
3) There is a solid solution β(Ti, Zr) which dissolves up to 16.3% Al. The maximum solubilities of Zr in Ti3Al and TiAl reach up to 17.9% and 4.0%, respectively.
4) The isothermal section consists of 16 single- phased regions, 27 two-phased regions and 14 three- phased regions. No ternary phase was detected.
References
[1] BAYRAKTAR E, BATHIAS C, XUE H Q, TAO H. On the giga cycle fatigue behaviour of (α2-Ti3Al and γ-TiAl) alloy [J]. International Journal of Fatigue, 2004, 26(2): 1263-1275.
[2] KRUZIC J J, CAMPBELL J P, RITCHIE O. On the fatigue behaviour of γ-based titanium aluminides: Role of small cracks [J]. Acta Mater, 1999, 47(3): 801-816.
[3] NIEH T G, HSIUNG L M, WADSWORTH J. Superplastic behavior of a powder metallurgy TiAl alloy with a metastable microstructure [J]. Intermetallics, 1999, 7: 163-170.
[4] WANG Yan, LIU Yong, YANG Guang-yu, LI Hui-zhong, TANG Bei. Microstructure of cast γ-TiAl based alloy solidified from β phase region [J]. Transactions of Nonferrous Metals Society of China, 2011, 21: 215-222.
[5] DENG Tai-qing, YE Lei, SUN Hong-fei, HU Lian-xi, YUAN Shi-jiang. Development of flow stress model for hot deformation of Ti-47%Al alloy [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(S2): s308-s314.
[6] CHEN Wei, GUAN Ying-ping, WANG Zhen-hua. Hot deformation behavior of high Ti6061Al alloy [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 369-377.
[7] QIU Cong-zhang, LIU Yong, HUANG Lan, ZhANG Wei, LIU Bin, LU Bin. Effect of Fe and Mo additions on microstructure and mechanical properties of TiAl intermetallics [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 521-527.
[8] KE Y, DUAN H, SUN Y. Effect of yttrium and erbium on the microstructure and mechanical properties of Ti-Al-Nb alloys [J]. Materials Science and Engineering A, 2010, 528: 220-225.
[9] FOX-RABINOVICH G S, WILKINSON D S, VELDHUIS S C, DOSBAEVA G K, WEATHERLY G C. Oxidation resistant Ti-Al-Cr alloy for protective coating applications [J]. Intermetallics, 2006, 14: 189-197.
[10] JIANG X J, ZHOU Y K, FENG Z H, XIA C Q, TAN C L, LIANG S X. Influence of Zr content on β-phase stability in α-type Ti-Al alloys [J]. Materials Science and Engineering A, 2015, 639: 407-411.
[11] FAN G J, SONG X P, QUAN M X, HU Z Q. Mechanical alloying and thermal stability of Al67Ti25M8 (M=Cr, Zr, Cu) [J]. Materials Science and Engineering A , 1997, 231: 111-116.
[12] BELOV N A, ALABIN A N, MATVEEVA I A, ESKIN D G. Effect of Zr additions and annealing temperature on electrical conductivity and hardness of hot rolled Al sheets [J]. Transactions of Nonferrous Metals Society of China, 2015, 25: 2817-2826.
[13]  X Y, GUO E J, ROMETSCH P, WANG L J. Effect of one-step and two-step homogenization treatments on distribution of Al3Zr dispersoids in commercial AA7150 aluminium alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2645-2651.
X Y, GUO E J, ROMETSCH P, WANG L J. Effect of one-step and two-step homogenization treatments on distribution of Al3Zr dispersoids in commercial AA7150 aluminium alloy [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2645-2651.
[14] WANG F, QIU D, LIU Z l, TAYLOR J, EASTON M, ZHANG M X. Crystallographic study of Al3Zr and Al3Nb as grain refiners for Al alloys [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 2034-2040.
[15] HARI KUMAR K C, WOLLANTS P, DELACY L. Thermodynamic assessment of the Ti-Zr system and calculation of the Nb-Ti-Zr phase diagram [J]. Journal of Alloys and Compounds, 1994, 206: 121-127.
[16] WANG T, JIN Z P, ZHAO J C. Thermodynamic assessment of the Al-Zr binary system [J]. Journal of Phase Equilibria, 2001, 22: 544-551.
[17] WITUSIEWICZ V T, BONDAR A A, HECHT U, REX S, VELIKANOVA T Y. The Al-B-Nb-Ti system: III. Thermodynamic re-evaluation of the constituent binary system Al-Ti [J]. Journal of Alloys and Compounds, 2008, 465: 64-77.
[18] YANG F, XIAO F H, LIU S G, DONG S S, HUANG L H, CHEN Q. Isothermal section of Al-Ti-Zr ternary system at 1273 K [J]. Journal of Alloys and Compounds, 2014, 585: 325-330.
[19] GHOSH G, ASTA M. First-principles calculation of structural energetics of Al-TM (TM = Ti, Zr, Hf) intermetallics [J]. Acta Materialia, 2005, 53: 3225-3252.
[20] KODENSTOV A A, F. BASTIN G, J J. van LOO F. Application of diffusion couples in phase diagram determination [C]//ZHAO Ji-chen, editor. Methods for Phase Diagram Determination. Oxford: Elsevier Science Ltd., 2007: 222-245.
Al-Ti-Zr三元系1073 K 等温截面的测定
吕凯丽,杨 冯,谢止云,刘华山,蔡格梅,金展鹏
中南大学 材料科学与工程学院,长沙 410083
摘 要:采用合金样与扩散偶相结合的方法,利用电子探针射线显微分析(EPMA)对Al-Ti-Zr三元系的1073 K等温截面的相关系进行实验测定。结果表明:截面中存在一个Al含量(摩尔分数)达到16.3%的β(Ti,Zr)固溶体。Ti和Zr可以在大多数Al-Zr和Ti-Al二元中间化合物中相互取代。其中,Zr在Ti3Al和TiAl中的最大固溶度分别达到17.9% 和 4.0%。该等温截面共包含16个单相区,27个两相区和14个三相平衡,没有检测到三元化合物。
关键词:Al-Ti-Zr 三元系;相平衡;扩散偶; 固溶度
(Edited by Yun-bin HE)
Foundation item: Project (51171210) supported by the National Natural Science Foundation of China; Project (2014CB6644002) supported by the Major State Basic Research Development Program of China
Corresponding author: Hua-shan LIU; Tel: +86-731-88876735; Fax: +86-731-88876692; E-mail: hsliu@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64436-9
Abstract: Through alloy sampling combined with diffusion triple technique, phase equilibria in Al-Ti-Zr ternary system at 1073 K were experimentally determined with electron probe microanalysis (EPMA). Experimental results show that there is a solid solution β(Ti,Zr) which dissolves Al up to 16.3% (mole fraction). Ti and Zr can substitute each other in most Ti-Al and Al-Zr binary intermediate phases to a certain degree while the maximum solubility of Zr in Ti3Al and TiAl reaches up to 17.9% and 4.0% (mole fraction), respectively. The isothermal section consists of 16 single-phased regions, 27 two-phased regions and 14 three-phased regions. No ternary phase was detected.


