
Deposition of electroless Ni-P/Ni-W-P duplex coatings on
AZ91D magnesium alloy
CHEN Xiao-ming(陈晓明), LI Guang-yu(李光玉), LIAN Jian-she(连建设)
Key Laboratory of Automobile Materials, Ministry of Education, College of Materials Science and Engineering,
Jilin University, Changchun 130025, China
Received 12 June 2008; accepted 5 September 2008
Abstract:
The electroless Ni-P/Ni-W-P duplex coatings were deposited directly on AZ91D magnesium alloy by an acid-sulfate nickel bath. Nickel sulphate and sodium tungstate were used as metal ion sources and sodium hypophosphite was used as reducing agent. The coating was characterized for its structure, morphologies, microhardness and corrosion properties. The presence of dense and coarse nodules in the duplex coatings was observed by SEM and EDS. Tungsten content in Ni-P/Ni-W-P alloy is about 0.65%(mass fraction) and the phosphorus content is 8.18%(mass fraction). The microhardness of the coatings is 622 VHN. The coating shows good adhesion to the substrate. The results of electrochemical analysis, the porosity and the immersion test show that Ni-P/Ni-W-P duplex coatings possess noble anticorrosion properties to protect the AZ91D magnesium alloy.
Key words:
corrosion resistance; electroless deposition; Ni-W-P alloy; acid-sulfate nickel;
1 Introduction
Magnesium has high strength-to-mass ratio with density of 1.74 g/cm3. Magnesium also has high thermal conductivity, high dimensional stability and good electromagnetic shielding characteristics, which make it valuable in a number of structural applications including aerospace, electronics, computer parts and automobile fields[1-3]. However, the application of magnesium alloys was limited due to the undesirable properties including poor corrosion and wear resistance. Corrosion and wear resistance of magnesium alloys were often enhanced by means of surface coatings or treatments. The corrosion of magnesium alloys depended on their metallurgy and environmental factors[3-5]. Since magnesium is one of the most electrochemically active metal, ordinary coatings, such as nickel, copper and zinc coatings, are cathodic to the magnesium alloy substrate and can only provide a physical barrier against the corrosion attack of substrate. So, any coatings on magnesium alloys should be uniform, adherent and pore-free as possible[6].
Among various surface techniques, electroless deposition like Ni-P alloy deposits is considered an effective method to modify the physical and chemical properties of the substrates due to their good corrosion resistance and high hardness (high wear resistance)[7], and it is thought to be the simplest and most economic method to finish steel, aluminum, copper, plastics and many other materials. The magnesium alloy is extremely susceptible to galvanic corrosion that pits severely on the metal, resulting in an unattractive appearance as well as decreased mechanical properties[8-9]. In the limited reports on the electroless Ni-P plating on the magnesium[3, 10], the substrates needed be etched first in a solution of chromate and nitric acid and then soaked in HF solution to form a conversion film before electroless nickel deposition. And the nickel ions were generally provided by basic nickel carbonate in the bath, which increased the cost and required careful control of the solutions.
Some ternary nickel based alloys are developed to further improve the properties of binary systems by adding the second metal salts into nickel solution to meet some special demands. Codeposition of W element in binary Ni-P deposit has been of considerable interest because of its unique properties, such as excellent corrosion-resistant, wear-resistant, electrical-resistant and high temperatures resistant. The corrosion resistances of electroless ternary Ni-W-P and Ni-W-Co alloys with various structures are higher than that of Ni-P deposits[11-12] and the annealed ternary Ni-W-P alloys has an even higher corrosion resistance than that of their amorphous counterpart due to the formation of a dense tungsten oxide film on the surface during annealing process[11]. Recently, the electroless Ni-P coating on AZ91D magnesium alloy was deposited [13] using nickel sulfate as nickel sources after the alloy was pickled in an acid solution of chromium oxide and activated in HF solution to form MgF2 film. The use of duplex-layer or multi-layer coatings with different corrosion potential Ecorr was an effective solution to improve the corrosion-resistance of the anodic substrate. The electroless Ni-P alloy coatings with different phosphorus contents have different electrochemical properties[14].
Based on the above mentioned works, the duplex coatings of Ni-P/Ni-W-P were deposited on AZ91D magnesium alloy by electroless deposition from the nickel sulfate solutions. The Ni-P coating on the magnesium alloy had higher phosphorus content in order to improve its corrosion potential. The difference in corrosion potential between Ni-P and Ni-W-P will improve the local corrosion environment, and the electroless Ni-P/Ni-W-P duplex coatings could be regarded as an electrochemical protection coating to AZ91D magnesium alloy against the corrosion of severe environments. The duplex coating is studied for its structures, morphologies, microhardness and corrosion characteristics.
2 Experimental
The substrate was AZ91D die cast magnesium alloy with size of 20 mm×20 mm×3 mm. The chemical composition of the alloy is listed in Table 1. The technical flow chart of the electroless deposition on the AZ91D magnesium alloy is listed in Table 2. The samples were cleaned thoroughly by deionized water as quickly as possible between any two steps of the treatments.
Table 1 Compositions of AZ91D magnesium alloy (mass fraction, %)

Table 2 Technical conditions of Ni-P/Ni-W-P duplex coating on AZ91D magnesium alloy

The pretreatment process plays a very important role in getting a good protective coating on magnesium and its alloys. The substrate was cleaned in alkali solution to remove soils or greases on the surface of magnesium alloy. But such ways to clean are not suitable for removing oxide[15], so after alkaline cleaning, the samples were etched in chromic acid and nitric acid solution to remove any oxide layer or other chemical coatings not fully removed by alkaline cleaning. Since, AZ91D Mg alloy consisted of primary α-Mg grains surrounded by a eutectic mixture of α and β-Mg17Al12[3], creating an equipotentialized film (MgF2) on the magnesium alloy surface is the next fluoride activation treatment. It is an indispensable step of pretreatments in the processing of the electroless plating on magnesium alloys.
The surface observation and element analysis of the coatings were realized by SEM (JSM-5310, Japan Electronics) and EDS (INC250). The structure of as-deposited Ni-W-P alloy was analyzed by X-ray diffractometer (XRD, Rigaku Dymax, Japan) with Cu Kα radiation (γ = 0.154 178 nm) and monochromator at 50 kV and 300 mA with the scanning rate of 4(°)/min and step of 0.02°.
In order to evaluate the porosity of the duplex coatings on magnesium alloy, a porosity test was proposed. The method was described in Ref.[8] and the porosity of coating was evaluated relatively by the ratio of red spot area on the filter paper. The immersion test in 10% HCl solution at room temperature was carried out for the duplex coatings on AZ91D magnesium alloy. Time interval between the start of the acid immersion test and the first hydrogen bubble arising from the coating surface was recorded and used to evaluate the corrosion resistance of the coatings on magnesium alloy[14]. In order to further evaluate the corrosion resistance and possible passivation behavior, electrochemical measurements in 3% NaCl aqueous solution were performed on an electrochemical analyzer (LK98C, Tianjin, China). Tafel plot was transformed from the recorded data and the corrosion current density (Jcorr) was determined by extrapolating the straight-line section of the anodic and cathodic Tafel curves.
The hardnesses of magnesium alloy before and after electroless deposition were evaluated by a HXD-1000 microhardness tester with Vickers indenter, employing a load of 200 g for 15 s. Five readings were taken on the deposit, and the values were then averaged. The thickness of these deposits was kept approximately at 20 μm. From Table 2, the thicknesses of the electroless Ni-P coating and the Ni-P/Ni-W-P coatings on the AZ91D magnesium alloy are about 10 μm and 20 μm, respectively.
3 Results and discussion
3.1 Compositions and morphologies of coatings
Fig.1 shows the XRD patterns taken on the AZ91D magnesium alloy substrate, the electroless Ni-P coating and the Ni-P/Ni-W-P coating on the AZ91D magnesium alloy, respectively. It can be seen from Fig.1 that, after electroless plating, the AZ91D magnesium substrate is fully covered by the as-plated electroless Ni-P alloy (see Fig.1(b)). The diffraction patterns of the Ni-P alloy have a single peak corresponding to the (111) plane of a face-centered cubic (fcc) nickel, which consists with the phosphorus contents of 3.70% determined by EDS. This pattern indicates that the structure of the as-deposited Ni-P coating is a mixture of amorphous and nanocrystalline nickel[16]. While the diffraction pattern of the Ni-P/Ni-W-P deposits shown in Fig.1(c) has only a single very broad peak at 2θ = 44.88?, an amorphous structure is observed for the Ni-W-P coating. The composition of the electroless Ni-P and Ni-P/Ni-W-P coatings determined by EDS are listed in Table 3. Codeposition of the tungsten results in ternary Ni-W-P coating with phosphorus content of 8.18% and tungsten content of 0.65%, respectively. The major constituent, nickel, is autocatalytically active during the deposition process, while the reduction of the second metal, tungsten, will be determined by its electrochemical potential as well as its catalytic activity during the deposition process[17].
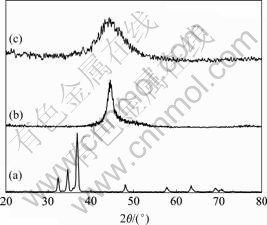
Fig.1 XRD patterns of deposition on AZ91D magnesium alloys at different steps: (a) Substrate of AZ91D magnesium alloys; (b) Ni-P deposition; (c) Ni-W-P deposition
Table 3 Compositions of electroless Ni-P and Ni-P/Ni-W-P coatings determined by EDS (mass fraction, %)

The morphologies of electroless Ni-P coating and Ni-W-P deposition on AZ91D Mg alloys are shown in Fig.2. Fig.2(a) shows the surface morphology of the Ni-P deposit on the AZ91D magnesium alloy, and Fig.2 (b) shows the subsequent Ni-W-P deposit on it. The later layer shows the typical spherical nodular structure, which is more round and compact than the former. Fig.2(c) shows the morphology of the cross-section of the double Ni-P/Ni-W-P coatings. The interface between the two deposits can be clearly seen. There are some pores in the Ni-P coating, which may result from the evaluation the hydrogen during the previous electroless Ni-P depositing. While in the Ni-W-P layer, no pore is found on the cross-section morphology. It seems that the coatings are connected closely to the substrate shown by the cross section morphology. The hardness of the as-deposited Ni-P/Ni-W-P coatings is about 622 VHN, which is slightly higher than that of the as-deposited Ni-P coating on magnesium alloy (approximately 570 VHN) and far higher than that of the AZ91D magnesium alloy substrate (about 100 VHN).
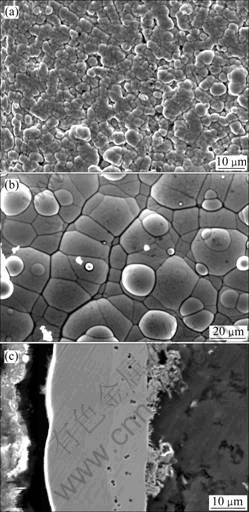
Fig.2 Morphologies of electroless Ni-P coating and Ni-P/Ni-W-P deposition on AZ91D Mg alloys: (a) Ni-P electroless; (b) Electroless Ni-W-P deposition on Ni-P coating; (c) Cross-section of Ni-P/Ni-W-P coating
3.2 Corrosion characteristics of coatings
The nickel/Mg system is a classical example of cathodic coating on an anodic substrate. Hence, the porosity in the coating might influence the corrosion behavior and service lifetime of the electroless nickel-plated magnesium[3]. The porosity of the Ni-P/Ni-W-P coatings was estimated by the porosity test proposed in the experiment part and the results as a function of the Ni-W-P coating thickness are shown in Fig.3. From Fig.3, the porosity decreases rapidly with the increase of the thickness of Ni-W-P. When the thickness reaches to about 8 μm, no porosity is found on the tested coatings. This observation shows that the tested areas are thick enough and pores are free to protect the substrate from corrosion. Thus, the electroless coating with low porosity is effective to protect the substrate from the corrosion of Cl-.
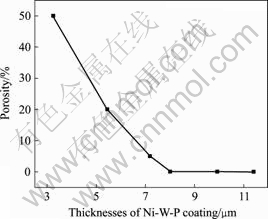
Fig.3 Relationship between porosity and deposition thickness of electroless Ni-W-P coatings
The immersion test results in the 10% HCl solution for the Ni-W-P coating with different thickness are shown in Fig.4. The time interval between the start of the test and the first hydrogen gas bubble arising from the coating surface is used to donate the corrosion resistance of the coatings on magnesium or magnesium alloys. When the thickness of Ni-W-P exceeds 15 μm, the corrosion-resistance of duplex coatings increases distinctly. There are no hydrogen gas bubbles arising from the 19 μm Ni-W-P coating on AZ91D magnesium alloy after immersed in the 10% HCl solution for 170 min. Therefore, it can be further confirmed that the duplex Ni-P/Ni-W-P coatings on magnesium alloy exhibit good anti-corrosion performance.

Fig.4 Relationship between corrosion time in 10% HCl solution and thickness of electroless Ni-W-P coatings
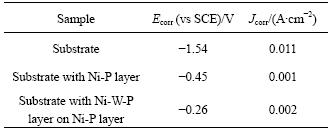
Fig.5 shows the electrochemical polarization curves for the AZ91D magnesium alloy substrate (curve 1) and the electroless Ni-P/Ni-W-P coatings (curve 3) in 3% NaCl aqueous solution at room temperature. For comparison, the polarization curve of the direct electroless Ni-P on the substrate (curve 2) is also shown in Fig.5. The corrosion potential and corrosion current density of the substrate and the coatings obtained from the electrochemical polarization curves are listed in Table 4. According to the polarization curves, no defects are assumed to be present in the tested samples. The cathode reaction in the polarization curves corresponds to the evolution of the hydrogen, and the anodic polarization curve is the most important features related to the corrosion resistance[18-19]. Both the Ni-P and Ni-P/Ni-W-P coatings show great positive shifts in corrosion potential and evident decreases in corrosion current density comparing with their magnesium alloy substrate (curve 1). The corrosion potential Ecorr of the Ni-P/Ni-W-P coatings on the substrate is more positive (-0.26 V) than that (-1.54 V) of the substrate. The corrosion current density Jcorr decreases evidently from 11 mA/cm2 of the substrate to 2 mA/cm2 of the electroless Ni-P/Ni-W-P layers. As for the Ni-P layer on the substrate, the corrosion potential Ecorr is shifted positively to about 1.09 V compared with that of the substrate, and the corrosion current density Jcorr decreases to about 1 mA/cm2. Therefore, the Ni-W-P layer on the AZ91D magnesium alloy possesses more positive corrosion potential Ecorr and exhibits much lower corrosion current density Jcorr. The polarization results indicate that the Ni-P/Ni-W-P duplex coatings should exhibit high corrosion resistance, which is in accordance with the porosity and immersion corrosion tests. When the porosity of the duplex nickel coatings reduces, the corrosion potential Ecorr will become positive and the corrosion current Jcorr will become smaller[18].
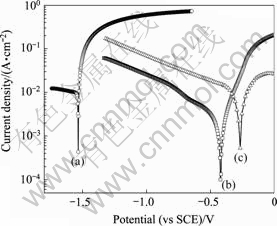
Fig.5 Polarization curves of AZ91D magnesium alloy substrate(a), substrate with Ni-P coating(b) and Ni-P/Ni-W-P duplex coating(c) in 3% NaCl aqueous solution
Table 4 Corrosion potential and corrosion current density obtained from electrochemical polarization curves
In summary, according to the porosity test, the acid immersion test and polarization curves measurements, the corrosion resistance of the electroless Ni-P/Ni-W-P coatings is much higher than that of the electroless Ni-P coating on AZ91D magnesium alloy, and the double coatings with thickness more than 20 μm is enough to protect the magnesium alloy substrate from corrosion.
4 Conclusions
1) The electroless of Ni-P/Ni-W-P alloy is deposited from a sulfate nickel bath on the Ni-P coating on AZ91D magnesium alloy.
2) The tungsten content in Ni-P/Ni-W-P alloy is about 0.65% and the phosphorus content is 8.18%. The duplex coatings show typical dense nodular structure. The hardness of the coatings is about 622 VHN, which is far higher than that of the AZ91D magnesium alloy substrate.
3) The dense and pore-free microstructure makes the coatings possessing noble anticorrosion properties on the AZ91D magnesium alloy substrate.
References
[1] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys-A critical review [J]. Journal of Alloys and Compounds, 2002, 336(1/2): 88-113.
[2] LIU Z M, GAO W. A novel process of electroless Ni-P plating with plasma electrolytic oxidation pretreatment [J]. Applied Surface Science, 2006, 253(5): 2988-2991.
[3] AMBAT R, ZHOU W. Electroless nickel plating on AZ91D magnesium alloy: Effect of substrate microstructure and plating parameters [J]. Surface and Coatings Technology, 2004, 179(2/3): 124-134.
[4] UMEHARA H, TAKAYA M, TERAUCHI S. Chrome-free surface treatments for magnesium alloy [J]. Surface and Coatings Technology, 2003, 169/170(1): 666-669.
[5] DABALA M, BRUNELLI K, NAPOLITANI E, MAGRINI M. Cerium-based chemical conversion coating on AZ63 magnesium alloy [J]. Surface and Coatings Technology, 2003, 172(2/3): 227-232.
[6] LIAN J S, LI G Y, NIU L Y, GU C D, JIANG Z H, JIANG Q. Electroless Ni-P deposition plus zinc phosphate coating on AZ91D magnesium alloy [J]. Surface and Coatings Technology, 2006, 200(20/21): 5956-5962.
[7] HAJDU J B. Electroless plating [J]. Plating and Surface Finishing, 1996, 83(9): 29-33.
[8] MALLORY G O, HAJDU J B. Electroless plating: Fundamentals and applications [M]. Orlando, FL: AESF Publishing, 1990: 261-265.
[9] YAN H. New techniques in electroless Ni and composite plating [M]. Beijing: National Defense Industry Press, 2001: 1-2. (in Chinese)
[10] SHARMA A K, SURESH M R, BHJRAJ H, NARAYNAMURTHY H, SAHU R P. Black anodizing of a magnesium-lithium alloy [J]. Metal Finishing, 1998, 96(4): 16-27.
[11] GAO Y, ZHANG Z J, ZHU M, LUO C P. Corrosion resistance of electrolessly deposited Ni-P and Ni-W-P alloys with various structures [J]. Materials Science and Engineering A, 2004, 381(1/2): 98-103.
[12] LU G J, ZANGARI G. Corrosion resistance of ternary Ni-P based alloys in sulfuric acid solutions [J]. Electrochimical Acta, 2002, 479(18): 2969-2979.
[13] GU C D, LIAN J S, LI G Y, NIU L Y. Electroless Ni-P plating on AZ91D magnesium alloy from a sulfate solution [J]. Journal of Alloys and Compounds, 2005, 391(1/2): 104-109.
[14] GU C D, LIAN J S, JIANG Z H. Multilayer Ni-P coating for improving the corrosion resistance of AZ91D magnesium alloy [J]. Advanced Engineering Materials, 2005, 7(11): 1032-1036.
[15] ZHANG W X, JIANG Z H, LI G Y, LIAN J S, JIANG Q. Electroless Ni-P/Ni-B duplex coatings for improving the hardness and the corrosion resistance of AZ91D magnesium alloy [J]. Applied Surface Science, 2008, 254(16): 4949-4955.
[16] GUO Z, KEONG K G, SHA W. Crystallization and phase transformation behaviour of electroless nickel phosphorus platings during continuous heating [J]. Journal of Alloys and Compounds, 2003, 358(1/2): 112-119.
[17] BALATAJU J N, RAJAM K S. Electroless deposition of Ni-Cu-P, Ni-W-P and Ni-W-Cu-P alloys [J]. Surface and Coatings Technology, 2005, 195(1/2): 154-161.
[18] LIAN J S, GU C D, NIU L Y, JIANG Z H, JIANG Q. Electroless Ni-P deposition plus zinc phosphate coating on AZ91D magnesium alloy [J]. Surface and Coatings Technology,2006, 200(20/21): 5956-5962.
[19] DONG H, SUN Y, BELL T. Enhanced corrosion resistance of duplex coatings [J]. Surface and Coatings Technology,1997, 90(1/2): 91-101.
Foundation item: Project (2004CB619301) supported by the National Basic Research Program of China; Project supported by the “985” Project of Jilin University, China; Project (2007KZ09) supported by the 2007 Science and Technology Support Plan of Changchun City, China
Corresponding author: LI Guang-yu; Tel: +86-431-85095875; Fax: +86-431-85095876; E-mail: guangyu@jlu.edu.cn


