
Preparation and osteoinduction of active micro-arc oxidation films on Ti-3Zr-2Sn-3Mo-25Nb alloy
YU Sen1, 2, YU Zhen-tao1, Gui WANG2, HAN Jian-ye1, MA Xi-qun1, Matthew S. DARGUSCH2
1. Northwest Institute for Nonferrous Metal Research, Xi’an 710016, China;
2. CAST Cooperative Research Centre, School of Mechanical and Mining Engineering,
The University of Queensland, Brisbane QLD 4072, Australia
Received 9 April 2010; accepted 5 July 2010
Abstract:
A layer of porous film containing Ca and P was prepared by the micro-arc oxidation method on the surface of a novel near β biomedical Ti-3Zr-2Sn-3Mo-25Nb alloy, and then NH2- active group was introduced to the films by activation treatment. The phase composition, surface micro-topography and elemental characteristics of the micro-arc oxidation films were investigated with XRD, SEM, EDS and XPS, and the osteoinduction of the micro-arc oxidation films was tested using the simulated body fluid immersion, the in-vitro osteoblast cultivation test and animal experiment. The results show that the oxide layer is a kind of porous ceramic intermixture and contains Ca and P. The films in the simulated body fluid can induce apatite formation, resulting in excellent bioactivity. The cell test discovers that osteoblasts can grow well on the surface of micro-arc oxidation films. And the Ti-3Zr-2Sn-3Mo-25Nb biomedical alloy coated with active porous calcium-phosphate films shows better osteoinduction in vivo.
Key words:
β titanium alloy; Ti-3Zr-2Sn-3Mo-25Nb alloy; osteoinduction; micro-arc oxidation; surface modification;
1 Introduction
Titanium and its alloys are widely used for dental and orthopedic implants because of their superior mechanical properties, excellent corrosion resistance and good biocompatibility; however, being bioinert, titanium and its alloys cannot directly bond to bone, and untreated alloys exhibit poor osteoinductive properties[1-2]. In order to promote direct attachment of surrounding hard tissue and to suppress the release of corrosion products into the human body, various surface modification techniques to synthesize the bioactive coatings such as calcium phosphates on the titanium and its alloys with the required mechanical properties have been given significant attention, such as plasma spraying, anodizing, sputtering, and sol-gel methods[3-5].
Out of all the available surface modification techniques, micro arc oxidation (MAO) is one of the most effective methods to modify the surface of titanium and its alloys for better biocompatibility and bioactivity[1, 3]. The MAO coatings usually exhibit good adhesion to substrates, and also exhibit a variety of different properties that depend on the composition and microstructure of the materials and processing parameters. The MAO film has a double structure composed of an inner barrier type layer and an outer porous layer. Thus the electrochemically formed oxide layers on titanium are both porous and also firmly adhere to the substrate. This is beneficial for the biological performance of the implants. Another advantage of the MAO process is that the oxide layer is formed uniformly on the metal surface with a complex geometry[5-8]. And several in vitro and in vivo studies have shown that micro-arc-oxidized surfaces have a higher early level of cell attachment and proliferation rates than the untreated Ti surface[6]. It was also shown that a micro-arc- oxidized surface had a greater number of osteoblasts with higher cell activity than bare Ti surface[3-7].
The near β Ti-3Zr-2Sn-3Mo-25Nb alloy, referred to as the TLM alloy, has been reported elsewhere[9-11]. The objective of this study is to identify the possibility of implanting calcium and phosphorus into an oxide film formed on this alloy by MAO followed by activation treatment so as to enhance biocompatibility and osteoinduction of the TLM alloy.
2 Materials and experimental methods
2.1 Materials
The TLM alloy samples (2 mm in diameter and 10 mm in thickness) were cut from 10 mm-thick hot-rolled sheet. Prior to anodizing, the samples were ground and polished with abrasive paper until a mirror finish was obtained. The samples were then etched in a mixture of hydrofluoric and nitric acids with volume fractions of 10% and 40% respectively for 5 s to remove the surface oxides, rinsed with distilled water and cleaned ultrasonically with acetone followed by propanol, and then dried in air at 40 °C.
2.2 Preparation and surface activation of MAO films
The TLM alloy samples were used as anodes, and stainless-steel plates were used as cathodes in an electrolytic bath containing 0.08 mol/L β- glycerophosphate disodium salt pentahydrate (C3H7Na2O6P·5H2O, β-GP) and 0.8 mol/L calcium acetate monohydrate ((CH3COO)2Ca·H2O, CA). The MAO process was conducted at a fixed applied voltage in the range of 250-500 V using a direct current pulse power supply with a pulse frequency, a duty circle, and a duration time set at 1000 Hz, 40% and 7 min, respectively. The system temperature was maintained below 50 °C by a water bath during the anodizing process. After the above MAO treatment, the samples were washed with distilled water and dried in the drying cabinet[12-13].
The films obtained using the above MAO process were submitted to grafting reactions in aqueous solution with the aid of the ceric ion technique using acrylamide (17%) in nitric acid and ceric ammonium solutions (0.04 and 0.006 mol/L, respectively) at 70 °C for 12 h under a stream of nitrogen. Samples were washed extensively with 0.02 mol/L NaOH followed by hot water[14-15].
2.3 Characterization of films
The analysis of the phase identification of the active MAO films on the TLM samples has been performed using X-ray diffractometer (Cu Kα, scanning rate of 0.03(°)/s, scanning angle of 20°-80°, deflection angle of 1°). The surface characteristics of the films were observed using a scanning electron microscope (JSM-6460, JEOL) and a quantative analysis of the chemical composition of the anodic layer formed on the surface of TLM samples was obtained using energy dispersive X-ray analyzer (AXIS ULTRA).
2.4 Immersion tests of samples in simulated body fluid
The samples were soaked in 50 mL SBF in a plastic vial and stored in an oven at 37 oC for 14 d, with the SBF being refreshed every two days. After immersion for a pre-determined period of time, the samples were removed from the SBF, washed with distilled water and then dried.
2.5 Cell culture test
MC3T3-E1 cells derived from mouse calvarium tissue were used as the immortalised cell-line. The culture medium contained 45% α-MEM (gibco), 45% MEM (gibco), 5% calf serum (eurobio) and 5% fetal bovine serum (eurobio). The medium also contained gentamicin (50 μg/mL) and amphotericin-B (250 μg/mL). 1×105 cm-2 growing cells contained in 1 mL culture medium were seeded in each well on the test samples and incubated at 37 °C and 5% CO2 atmosphere with 100% humidity. The cell morphology was assessed after culture for 2 d using SEM (JSM 6700, JEOL, USA)[16-17].
2.6 Surgical procedures and histology preparations
Six China white rabbits were prepared by intramuscularly injecting Ketamine (15 mg/kg) and diazepam (0.5 mg/kg). Two slightly oversized holes (2.1 mm in diameter), separated by about 10 mm apart, were made in the later cortex bone of one of the rabbit’s femurs. Two porous TLM cylinders were implanted in one leg of the rabbit and two bulk TLM cylinders were implanted in the other leg as a paired control. The rabbits were sacrificed with an air infection at 2, 4, 12 weeks post-operation. Before being killed, the rabbits had taken acheomycin (100 mg/(kg?d-1) for 6 d.
When the rabbits were killed, segments of the rabbit femur containing the implants were excised. All specimens were immediately immersed into pentadiol solution to fix the cell. The samples were dehydrated in serial concentrations of ethanol, fixed with osmic acid again, and finally sputter coated with gold for examination under SEM (JSM 6700, JEOL, USA).
3 Results and discussion
3.1 XRD and EDS analysis
The phase constituents of the oxide layers formed by the MAO process were characterized by XRD analysis, as shown in Fig.1. It can be seen that the oxidized layer was a mixture of TiO2 and a minor amount of amorphous phase with peaks of titanium. TiO2
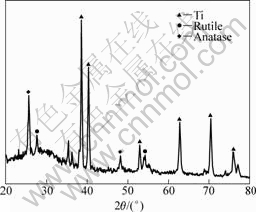
Fig.1 XRD pattern of film produced by MAO and activation treatment
was present in the form of anatase and rutile, but the intensity of the anatase TiO2 peaks was stronger than that of the rutile TiO2. No Ca- and P- containing phases were detected by XRD.
The chemical composition of the surface layer formed by the MAO process was determined using EDS analysis, as shown in Fig.2(a). The films formed by exposure to the Ca- and P-containing solution contain both Ca and P along with Ti and O. This implies that the elemental component in an electrolytic solution can be compounded into the films by using the MAO process[16-17].
During the MAO process, the initial amorphous TiOx passivating coating on the surface of the titanium alloy was broken by yielding micro arc discharge, and a discharge channel was formed. Thus, the electrolyte can

Fig.2 Composition variation of films produced by MAO and activation treatment: (a) EDS pattern; (b) SEM micrograph of cross-section; (c) Ca element distribution (EDS); (d) P element distribution (EDS); (e) Ti element distribution (EDS); (f) O element distribution (EDS)
flow into the discharge channel, and the titanium alloy substrate can contact the electrolyte. During the micro arc discharge process under an applied voltage, intense plasma physical and chemical reactions occur in the discharge channel, accompanied by high pressure and high temperature conditions. Under these conditions, Ti near the surface of the titanium alloy substrate participates in the subsequent reactions and can be oxidized to form TiO2. At the same time, Ca2+ and HPO42- or PO43- and OH- from the ionization of the CA, β-GP and H2O move to the anode easily in the electrolyte under the applied electric field respectively and are incorporated into the coatings [16]. For biomedical applications, Ca2+ and PO34- ions in the electrolyte enter the ceramic layer during the MAO process, so that the bioactivity of the titanium alloy increases[17-18].
Fig.2(b) shows not only SEM images of the cross-section of the coating, but also the sketch map of the line scan. Figs.2(c)-(f) show the primary elemental distributions from substrate to the MAO films. All the oxidized layers contain Ca and P as well as Ti and O. And all the elements show a graded distribution along the coating depth. By increasing the coating thickness, the Ca, O and P concentrations increase; while the Ti concentrations decrease. Actually, a coating with a graded structure is usually favored due to its mechanical stability and good match with the crystallographic structure and the thermal expansion coefficients between the coating and substrate[16-18].
3.2 Surface morphology of MAO films
The oxidized surface formed in the Ca- and P-containing electrolytic solution shows a porous microstructure with micron sized pores, as shown in Figs.3(a) and (b), which were relatively well separated and homogeneously distributed over the sample. During the early stage of the treatment, the formation of a barrier film was initiated by micro-arc oxidation in the electrolyte. By imposing a higher voltage, micro-arcing due to electrical breakdown of this dielectric layer became active and vigorous, resulting in pores across the titanium surface. A porous surface in implants is beneficial to bone tissue growth and enhances the anchorage of implants to the bone[18].
3.3 XPS analysis
Fig.4 shows the XPS results of micro-arc oxidatized films before and after surface modification. After surface activation of micro-arc oxidation films, elements of N appear on the surface of sample as active functional group, and the analytic spectrum explained that the new nitrogen come from amino function group (399.8 eV). At the same time, no nitrogen appears on the surface of
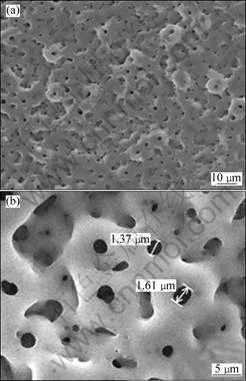
Fig.3 SEM surface morphologies of TLM titanium samples treated with MAO in Ca- and P-containing solution followed by activation: (a) Low magnification; (b) High magnification
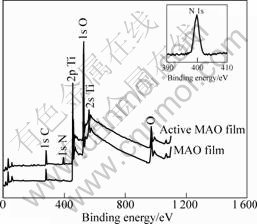
Fig.4 XPS patterns of TLM alloy before and after surface modification treatment
sample without surface activation.
3.4 Ca-P precipitation in SBF
The original MAO surface exhibited a porous microstructure. Those pores had a spherical morphology and are smooth and uniform, as shown in Fig.5(a). After the MAO treatment, the samples were soaked in SBF at 37 °C to induce the formation of bioactive apatite. SEM observation shows that a large amount of white scale-likeer immersed in th
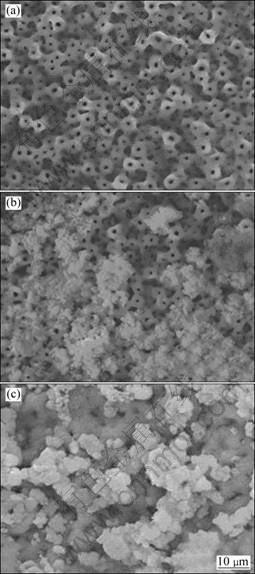
Fig.5 SEM images of original MAO surface (a), active MAO films soaked in SBF at 37 °C for 7 d (b) and 14 d (c)
particles appeared in some areas, such as pores and surface steps within 7 d, as shown in Fig.5(b). With an increase in soaking time up to 2 weeks, scale-like precipitates covered the whole porous surface of the samples, as shown in Fig.5(c). It is believed that the apatite nuclei were formed after approximately 14 d of immersion and they continued to grow, initially filling the pores, and then, spreading over the entire surface. The phase changes in the oxidized films during immersion in the SBF were investigated by XRD and are shown in Fig.6. However, apparently distinct apatite peaks appeared after 7 d of immersion. All these results confirm that apatite could be formed on the surface of the activate MAO films.
It has been widely reported that the essential requirement for an artificial material to bond to living bone is the formation of a bone-like apatite layer on its surface in the body environment. This in vivo apatite formation, also, can be reproduced using SBF. This means that the bioactivity of a material can be predicted from the apatite-forming ability of its surface after immersion in the SBF[16, 18].

Fig.6 XRD pattern of active MAO treated specimen after soaking in SBF at 37 °C for 7 d
In situ formation of the apatite on the surface of the active oxidized TLM in the SBF appears to be closely related to the Ca- and P-containing compounds. SBF is a metastable calcium phosphate solution supersaturated with respect to apatite. However, it has been reported that the barrier for the homogeneous nucleation of apatite is too high and a chemical stimulus is required in order to induce the heterogeneous nucleation of apatite from the SBF[5, 13]. The surface hydroxyl groups such as CaOH, TiOH, and COOH are known to be efficient inducers of apatite nucleation. In the oxidized films, CA was expected to undergo hydrolysis to form Ca2+, OH-, and TiO(OH)2 in the SBF. Hydroxylated titanium oxide is considered to be insoluble and yields a TiOH surface, which might act as a nucleation site. The hydrolysis of the Ca- and P-containing phases provides Ca2+, OH-, and HPO42- ionic species, which increase the local degree of supersaturation with respect to apatite near the surface. The provision of abundant TiOH groups and the enrichment of calcium and phosphate trigger the nucleation of apatite on the oxidized Ti surface. When the apatite nuclei are formed, they spontaneously grow at the expense of calcium and phosphate ions from the metastable supersaturated SBF solution[12, 14].
3.5 Osteoblast attachment and proliferation
The morphologies of the MC3T3-E1 cells grown on the porous specimens for 2 d have been assessed by SEM
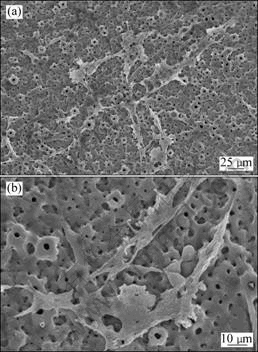
Fig.7 SEM images of osteoblasts grown on active porous films: (a) Low magnification; (b) High magnification
and results are shown in Figs.7(a) and (b). The cells were in close contact with the specimen and were well flattened over the substrate, as shown in Fig.7(a). In addition, lamellipodes and fine spicula-like pseudopodias and intercellular connections were clearly observed (Fig.7(b)), and the pseudopods were fastened to the pores and steps on the surface, indicating an active cell migration and good livelihood. The cell pseudopods pulled the pores and the ridges together.
The ease by which cells attach, spread and grow are important criteria when judging the biocompatibility of a biomaterial. Cell behavior on biomaterial surfaces depends upon four material related surface factors: surface composition, energy, roughness and topography. In this study, good cell proliferation appears to be mainly related to porous surface structure, the chemical composition and ![]() active group of the surface layers. The porous surface structure is generally beneficial to bone tissue growth and enhances the anchorage of implants to the bone. A highly porous structure may be valuable as a depot for bioactive constituents such as growth factors or bone morphogenic protein. In this study, the porous and rough surface produced by the MAO increased the cell attachment. This improvement was attributed to an increase in surface roughness. The chemical composition of the surface layers also plays a crucial role in the cell proliferation on the surface of the specimens. The Ca and P ions incorporated into the oxidation process strongly influence the cell response. In this study, after MAO treatment, the incorporation of Ca-containing compound into the oxide layer supplied more Ca2+ which bond to the negative ions of the protein, resulting in a proportional increase in cell differentiation and propagation. In addition, the amino which has been introduced onto the surface of the MAO films (Fig.4) is electronegative and this increases the hydrophilia, which assists the MC3T3-E1 cells to proliferate and differentiate[16-17].
active group of the surface layers. The porous surface structure is generally beneficial to bone tissue growth and enhances the anchorage of implants to the bone. A highly porous structure may be valuable as a depot for bioactive constituents such as growth factors or bone morphogenic protein. In this study, the porous and rough surface produced by the MAO increased the cell attachment. This improvement was attributed to an increase in surface roughness. The chemical composition of the surface layers also plays a crucial role in the cell proliferation on the surface of the specimens. The Ca and P ions incorporated into the oxidation process strongly influence the cell response. In this study, after MAO treatment, the incorporation of Ca-containing compound into the oxide layer supplied more Ca2+ which bond to the negative ions of the protein, resulting in a proportional increase in cell differentiation and propagation. In addition, the amino which has been introduced onto the surface of the MAO films (Fig.4) is electronegative and this increases the hydrophilia, which assists the MC3T3-E1 cells to proliferate and differentiate[16-17].
3.6 Hard tissue reaction analysis
Fig.8 shows the surface morphologies of implants after 2, 4, 12 weeks implantation. After two weeks, a little bulged cell, osteoplast, lamellar bone and collagen fibres can be observed on the bulk TLM implants (Fig.8(a)). While in the porous TLM group, a large number of bulged cells, organic matrix, osteoplast and trabecularism (woven bone) are present and a large number of collagen fibrous networks formed around the cells (Fig.8(d)). And in the following weeks (Figs.8(e) and (f)), proliferation of osteoblasts is very active on the surface of the porous TLM implants, indicating abundant growth of new bone on the porous TLM surface. After 12 weeks, almost all of the surface area on the activated porous TLM implants is directly covered with new bone (Fig.8(f)), and no fibrous tissue can be found at the interface. The modified surface provides good biological fixation to the surrounding tissue through bone tissue ingrowth into the porous network. But in the same time, only a little osteoid, architectural deformation and lamellar bone can be found on the surface of bulk TLM, having no specific characteristics of bone ingrowth (Figs.8(b) and (c)).
4 Conclusions
1) Micro-arc oxidation in Ca- and P-containing electrolytic solution subsequently was activated in an aminated solution, and active porous ceramic film containing Ca and P was prepared on the surface of the TLM alloy. The Ti, Ca, O and P elements show graded distribution along the coating depth.
2) The surface layers effectively induce apatite formation after a short time immersion in simulated body fluid. And the MC3T3-E1 cell proliferation on the surfaces of titanium alloy is good.
3) The results from hard tissue implantation indicate that the active surface porous TLM alloy exhibits good biocompatibility and better osteoinduction than unmodified TLM.
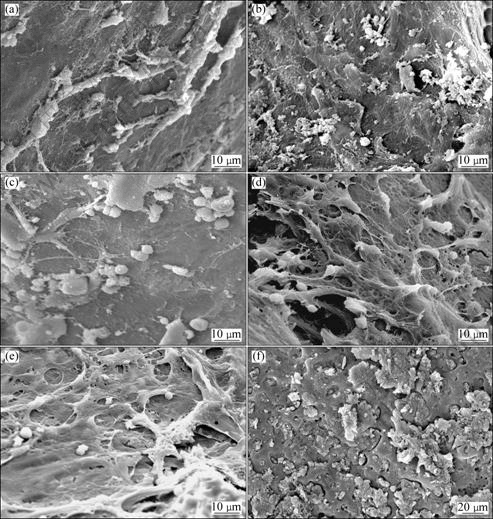
Fig.8 Morphologies of surface of implants at each stage after implantation, respectively: (a) TLM, 2 weeks; (b) TLM, 4 weeks; (c) TLM, 12 weeks; (d) Surface active porous TLM, 2 weeks; (e) Surface active porous TLM, 4 weeks; (f) Surface active porous TLM, 12 weeks
References
[1] LIU Xuan-yong, CHU P K, DING Chuan-xian. Surface modification of titanium, titanium alloys, and related materials for biomedical applications [J]. Materials Science and Engineering R, 2004, 47: 49-121.
[2] WILLIAMS D F. On the mechanisms of biocompatibility [J]. Biomaterials, 2008, 29: 2941-2953.
[3] GEETHA M, SINGH A K, ASOKAMANI R, GOGIA A K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review [J]. Progress in Materials Science, 2009(54): 397-425.
[4] ZAFFE D. Some considerations on biomaterials and bone [J]. Micron, 2005, 36: 583-592.
[5] PAITAL S R, DAHOTRE N B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies [J]. Materials Science and Engineering R, 2009, 66: 1-70.
[6] LI Xiao-ming, van BLITTERSWIJK C A, FENG Qing-ling, CUI Fu-zhai, WATARI F. The effect of calcium phosphate microstructure on bone-related cells in vitro [J]. Biomaterials, 2008, 29: 3306-3316.
[7] DAS K, BOSE S, AMIT B. Surface modifications and cell-materials interactions with anodized Ti [J]. Acta Biomaterialia, 2007, 3: 573-585.
[8] ZHENG C Y, LI S J, TAO X J, HAO Y L, YANG R. Surface modification of Ti-Nb-Zr-Sn alloy by thermal and hydrothermal treatments [J]. Materials Science and Engineering C, 2009, 29: 1245-1251.
[9] YU Zhen-tao, ZHOU Lian. Influence of martensitic transformation on mechanical compatibility of biomedical β type titanium alloy TLM [J]. Materials Science and Engineering A, 2006, 438-440: 391-394.
[10] YU Zhen-tao, WANG Gui, MA Xi-qun, DARGUSCH M S, HAN Jian-ye, YU Sen. Development of biomedical near β titanium alloys [J]. Materials Science Forum, 2009, 618-619: 303-306.
[11] YU Z, WANG G, MA X, ZHANG Y, DARGUSCH M S. Shape memory characteristics of a near β titanium alloy [J]. Materials Science and Engineering A, 2009, 513-514: 233-238.
[12] HUANG J, BEST S M, BONFIELD W, BUCKLAND T. Development and characterization of titanium-containing hydroxyapatite for medical applications [J]. Acta Biomaterialia, 2010, 6: 241-249.
[13] LI Yan, LEE I S, CUI Fu-zhai, CHOI S H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium [J]. Biomaterials, 2008, 29: 2025-2032.
[14] YU Sen, YU Zhen-tao, GUI Wang, DARGUSCH M S, ZHANG Ming-hua. Evaluation of haemocompatibility of TLM titanium alloy with surface heparinization [J]. Rare Metal Materials and Engineering, 2009, 38: 0384-0388.
[15] MICHANETZIS G P A, KATSALA N, MISSIRLIS Y F. Comparison of haemocompatibility improvement of four polymeric biomaterials by two heparinization techniques [J]. Biomaterials, 2003, 24: 677-688.
[16] KIM D Y, KIM M, KIM H E, KOH Y H, KIM H W, JANG J H. Formation of hydroxyapatite within porous TiO2 layer by micro-arc oxidation coupled with electrophoretic deposition [J]. Acta Biomaterialia, 2009, 5: 2196-2205.
[17] HUANG Yong, WANG Ying-jun, NING Cheng-yun, NAN Kai-hui, HAN Yong. Hydroxyapatite coatings produced on commercially pure titanium by micro-arc oxidation [J]. Biomed Mater, 2007, 2: 196-201.
[18] LI Long-hao, KONG Y M, KIM H W, KIM Y W, KIM H E, HEO S J, KOAK J Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation [J]. Biomaterials, 2004, 25: 2867-2875.
Ti-3Zr-2Sn-3Mo-25Nb合金表面活性微弧氧化膜的制备及其骨诱导性
余 森1, 2,于振涛1,Gui WANG2,韩建业1,麻西群1,Matthew S. DARGUSCH2
1. 西北有色金属研究院 西安 710016;
2. CAST Cooperative Research Centre, School of Mechanical and Mining Engineering,
The University of Queensland, Brisbane QLD 4072, Australia
摘 要:通过微弧氧化法在新型医用近β钛合金Ti-3Zr-2Sn-3Mo-25Nb表面制备一层含Ca、P多孔薄膜,再将其在胺基化溶液中活化处理以在薄膜表面引入![]() 。借助XRD、SEM和EDS研究该多孔复合薄膜的组成和表面形貌,并通过模拟体液浸泡实验、体外细胞培养实验和动物体内植入实验研究经上述表面改性处理后的Ti-3Zr-2Sn-3Mo-25Nb合金的骨诱导活性。结果表明:该薄膜主要由金红石型TiO2和锐钛矿型TiO2组成,是一种含有Ca、P的陶瓷混合物;薄膜在模拟体液中具有很好的生物活性,成骨细胞能够很好地在薄膜上分化、生长;表面覆膜处理的Ti-3Zr-2Sn-3Mo-25Nb合金的体内骨诱导活性优于未处理的Ti-3Zr-2Sn-3Mo-25Nb合金的。
。借助XRD、SEM和EDS研究该多孔复合薄膜的组成和表面形貌,并通过模拟体液浸泡实验、体外细胞培养实验和动物体内植入实验研究经上述表面改性处理后的Ti-3Zr-2Sn-3Mo-25Nb合金的骨诱导活性。结果表明:该薄膜主要由金红石型TiO2和锐钛矿型TiO2组成,是一种含有Ca、P的陶瓷混合物;薄膜在模拟体液中具有很好的生物活性,成骨细胞能够很好地在薄膜上分化、生长;表面覆膜处理的Ti-3Zr-2Sn-3Mo-25Nb合金的体内骨诱导活性优于未处理的Ti-3Zr-2Sn-3Mo-25Nb合金的。
关键词:β钛合金;Ti-3Zr-2Sn-3Mo-25Nb合金;骨诱导性;微弧氧化;表面改性
(Edited by YANG Hua)
Foundation item: Project (2005CB623904) supported by the National Basic Research Program of China; Project (30770586) supported by the National Natural Science Foundation of China; Project (31011120049) supported by the Australia-China special fund, International Science Linkages Program co-supported by the Department of Innovation, Industry, Science and Research of Australia, and the Ministry of Science and Technology and National Science Foundation of China; Project (2010ZDKG-96) supported by the major Subject of “13115” Programs of Shaan’xi Province, China
Corresponding author: YU Sen; Tel: +86-29-86231084; E-mail: ninbrc@gmail.com
DOI: 10.1016/S1003-6326(11)60753-X


