- Abstract:
- 1 Introduction▲
- 2 Experimental▲
- 3 Results and discus...▲
- 4 Conclusions▲
- References
- Figure
- Fig. 1 XRD patterns of as-cast and spun alloys:
- Fig. 2 HRTEM micrographs and ED patterns of as-spun (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys:
- Fig. 3 SEM images (a, b, c, d) together with typical EDS spectra (e, f, g) of as-cast alloys:
- Fig. 4 DSC curves of as-spun Nd10 alloy
- Fig. 5 Evolution of hydrogen absorption quantities of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with time varying at 200 °C:
- Fig. 6 Evolution of values of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with spinning rate varying
- Fig. 7 Evolution of hydrogen desorption quantities of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with time varying at 250 °C:
- Fig. 8 Evolution of values of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with spinning rate varying
- Fig. 9 Evolution of capacity maintaining rate (Sn) together with hydrogen absorption capacity of as-spun (40 m/s) Nd0 and Nd10 alloys with cycle number
- Fig. 10 SEM images of granular morphologies of as-spun (40 m/s) Nd0 and Nd10 alloys before and after hydriding and dehydriding cycles:
- Fig. 11 XRD patterns of as-spun (40 m/s) Nd10 alloy before and after hydriding and dehydriding cycle
J. Cent. South Univ. (2016) 23: 2754-2762
DOI: 10.1007/s11771-016-3337-0

Effect of melt spinning on gaseous hydrogen storage characteristics of nanocrystalline and amorphous Nd-added Mg2Ni-type alloys
ZHANG Yang-huan(张羊换)1, 2, YUAN Ze-ming(袁泽明)2, YANG Tai(杨泰)2, QI Yan(祁焱)2,GUO Shi-hai(郭世海)2, ZHAO Dong-liang(赵栋梁)2
1. Key Laboratory of Integrated Exploitation of Baiyun Obo Multi-Metal Resources, Inner Mongolia University of Science and Technology, Baotou 014010, China;
2. Department of Functional Material Research, Central Iron and Steel Research Institute, Beijing 100081, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Nanocrystalline and amorphous Mg-Nd-Ni-Cu quaternary alloys with a composition of (Mg24Ni10Cu2)100-xNdx (x=0, 5, 10, 15, 20) were prepared by melt spinning technology and their structures as well as gaseous hydrogen storage characteristics were investigated. The XRD, TEM and SEM linked with EDS detections reveal that the as-spun Nd-free alloy holds an entire nanocrystalline structure but a nanocrystalline and amorphous structure for the as-spun Nd-added alloy, implying that the addition of Nd facilitates the glass forming in the Mg2Ni-type alloy. Furthermore, the degree of amorphization of the as-spun Nd-added alloy and thermal stability of the amorphous structure clearly increase with the spinning rate rising. The melt spinning ameliorates the hydriding and dehydriding kinetics of the alloys dramatically. Specially, the rising of the spinning rate from 0 (the as-cast was defined as the spinning rate of 0 m/s) to 40 m/s brings on the hydrogen absorption saturation ratio 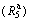 (a ratio of the hydrogen absorption quantity in 5 min to the saturated hydrogen absorption capacity) increasing from 36.9% to 91.5% and the hydrogen desorption ratio
(a ratio of the hydrogen absorption quantity in 5 min to the saturated hydrogen absorption capacity) increasing from 36.9% to 91.5% and the hydrogen desorption ratio 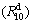 (a ratio of the hydrogen desorption quantity in 10 min to the saturated hydrogen absorption capacity) rising from 16.4% to 47.7% for the (x=10) alloy, respectively.
(a ratio of the hydrogen desorption quantity in 10 min to the saturated hydrogen absorption capacity) rising from 16.4% to 47.7% for the (x=10) alloy, respectively.
Key words:
1 Introduction
Hydrogen storage is one of the important components of hydrogen economy. Of the available ways of hydrogen storage, metal hydride systems are regarded to be more accurate, efficient and safe, representing the frontiers of technology [1]. Among these materials, Mg and Mg-based compounds are looked upon as one of the most promising hydrogen storage materials applied to hydrogen fuel cell because of the high hydrogen capacity of the hydrides, e.g. 7.6% (mass fraction) for MgH2, 3.6% for Mg2NiH4, 4.5% for Mg2CoH5 and 5.4% for Mg2FeH6 compared to the well established low temperature metal hydrides such as LaNi5 (1.4%) or TiFe and TiMn (1.8%) at a lower cost [2-3]. On the other hand, although the theoretical hydrogen capacities of other complex hydrides such as LiBH4 and NaAlH4 are higher than that of Mg2NiH4 [2, 4], the use of these complex hydrides for hydrogen storage is severely nullified because of kinetics and thermodynamics limitations [2]. In fact, the Mg2Ni-type alloys have been viewed as one of the most promising negative electrodes in Ni-MH batteries [5] or hydrogen storage materials applied in hydrogen fuel cell vehicle because of their high theoretical electrochemical capacity (about 1000 mA·h/g) and gaseous hydrogen absorption capacity (3.6%) for Mg2NiH4 [6-7]. However, the practical application of the Mg-based alloys is frustrated by some inherent disadvantages such as relatively high dehydrogenation temperature and sluggish hydriding/ dehydriding kinetics as hydrogen storage materials of on-board use. In spite of facing enormous challenges, the researchers in this field still persist in the firm confidence to improve the properties of the alloys and have made breakthrough progress. It is universally agreed that alloying and microstructure modification are effective approaches for improving the hydriding properties [8]. Particularly, the partial substitution of some elements (Y, La, Zr) for Mg and (Cu, Fe, V, Cr, Co) for Ni in Mg2Ni alloy makes the stability of the hydride decrease and the hydrogen desorption reaction easier [9-11]. It was documented that the hydriding and dehydriding kinetics of the Mg2Ni-type alloys are very sensitive to their structures [12]. Especially, their hydrogen storage properties are strongly affected by nanometer scale structures because of the thermodynamic and kinetic aspects. Heretofore, the high energy ball milling (HEBM) and melt-spinning technique are considered to be very effective methods to obtain an amorphous and/or nanocrystalline Mg2Ni alloy. However, some shortcomings of ball milling technology seem to be unavoidable: the milled materials are easily polluted by steel balls and air, even though in very good protection conditions. Also, the cycle stabilities of the milled Mg and Mg-based alloys are very poor owing to the vanishment of the metastable structures generated by ball milling during the multiple hydrogen absorbing and desorbing cycles [13]. On the contrary, the microstructure created by melt spinning displays much higher stability during the hydrogen absorbing and desorbing cycles compared with the microstructure generated by HEBM [14-15]. Meanwhile, the nanocrystalline and amorphous Mg-based alloys prepared by melt-spinning exhibit excellent hydriding characteristics, similar to the alloys produced by the HEBM process [16-17].
Our previous investigations have found that the substitution of La for Mg and M (M=Cu, Co, Mn) for Ni could improve the electrochemical and gaseous hydrogen storage performances of the Mg2Ni-type alloys dramatically [18-20]. Therefore, we expect that the joint addition of Cu and Nd combining with a proper melt spinning technique may ameliorate the hydrogen storage characteristics of the Mg2Ni-type alloy more markedly. To validate this, a systematical investigation about the effects of spinning rate on the structures and hydrogen storage performances of the (Mg24Ni10Cu2)100-xNdx (x= 0-20) electrode alloys has been performed, and some experimental results were provided.
2 Experimental
The compositions of the experimental alloys were (Mg24Ni10Cu2)100-xNdx (x=0, 5, 10, 15, 20). For convenience, the alloys were denoted with Nd content as Nd0, Nd5, Nd10, Nd15 and Nd20, respectively. The alloy ingots were prepared by using a vacuum induction furnace in a helium atmosphere at a pressure of 0.04 MPa to prevent Mg from volatilizing. A part of the as-cast alloys was re-melted and spun by melt spinning with a rotating copper roller cooled by water. The spinning rates used in the experiment were 10, 20, 30 and 40 m/s, respectively, which were approximately expressed by the linear velocity of the copper roller.
The phase structures of the as-cast and spun alloys were determined by X-ray diffraction (XRD) (D/max/ 2400). The diffraction, with the experimental parameters of 160 mA, 40 kV and 10 (°)/min, respectively, was performed with CuKα1 radiation filtered by graphite.
The thin film samples of the as-spun alloys prepared by using ion etching technology were observed by high resolution transmission electron microscope (HRTEM) (JEM-2100F, operated at 200 kV) and their crystalline states were ascertained by electron diffraction (ED).
A Philips SEM (QUANTA 400) linked with an energy dispersive spectrometer (EDS) was used for morphological characterization and chemical composition analysis of the as-cast alloys.
Thermal stability and crystallization of the as-spun alloys were studied by means of differential scanning calorimetry (DSC). Also, the DSC curve was measured in argon flow, and the samples were placed in an alumina crucible, heating temperature from 30 to 550 °C with the heating rate of 10 °C/min.
The hydrogen absorption and desorption kinetics of the alloys were measured by an automatically controlled Sieverts apparatus. The hydrogen absorption was conducted at 2 MPa hydrogen pressure (in fact, this pressure is the initial pressure of hydriding process) and 200 °C, and the hydrogen desorption at the pressure of 1×10-4 MPa and 250 °C.
3 Results and discussion
3.1 Microstructure characteristics
Figure 1 shows the XRD patterns of the as-cast and spun Nd0 and Nd10 alloys, from which it is found that the as-cast and spun Nd0 alloy displays a typical crystalline structure. Also, we note that the melt spinning renders the diffraction peaks of the Nd0 alloy evidently broaden, to be put down to the significant refinement of the grain. Differing from Nd0 alloy, the merged and broadened diffraction peaks of the as-cast Nd10 alloy after melt spun (10 m/s) indicate that the crystalline structure has transformed into an amorphous and nanocrystalline structure. With the spinning rate increasing, the diffraction peaks of the Nd10 alloy become broader and flatter, suggesting that the amount of amorphous phase is growing. It means that the addition of Nd facilitates the glass forming in the Mg2Ni-type alloy. Amorphous forming ability mainly related to the elements and atomic radius of alloy. The more elements and the lager atomic size difference there are, the stronger amorphous forming ability will be. The presence of Nd facilitates the formation of amorphous phase because the atomic size of Nd (0.264 nm) is lager than that of Mg (0.172 nm) and the atomic size difference is indeed the main factor for forming amorphous alloys [21]. This conclusion is also evidenced by TEM detections, as demonstrated in Fig. 2, from which there is an obvious evidence that the as-spun Nd0 alloy is strongly disordered and nanostructured, meanwhile, some crystal defects such as subgrains and grain boundaries can be seen clearly, and in its electron diffraction (ED) patterns sharp multi-haloes appear, corresponding to a nanocrystalline structure. Nevertheless, the as-spun Nd10 alloy differing from Nd0 alloy exhibits a clear feature of the nanocrystalline embedded in the amorphous matrix, and its electron diffraction patterns consist of broad and dull halo, indicating the existence of an amorphous structure, which corresponds with the XRD inspection as depicted in Fig. 1. Meanwhile, the phase components of the as-cast Nd0 and Nd10 alloys are analyzed by SEM, as illustrated in Fig. 3. Apparently, the addition of Nd results in the morphologies of the as-cast alloys changing obviously, namely the typical dendritic structure of the as-cast Nd0 alloy completely disappearing and some secondary phases emerging, which are Mg6Ni, Nd5Mg41 and NdNi determined by EDS patterns.
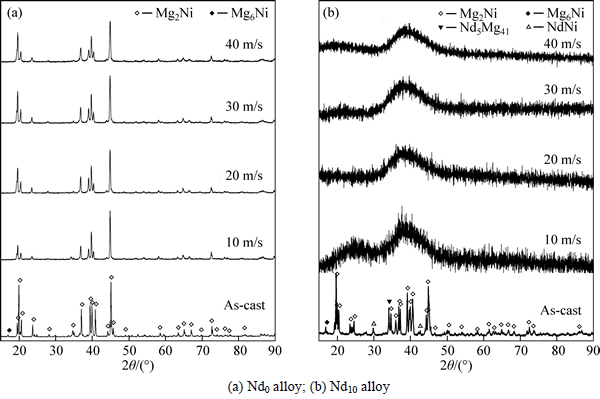
Fig. 1 XRD patterns of as-cast and spun alloys:
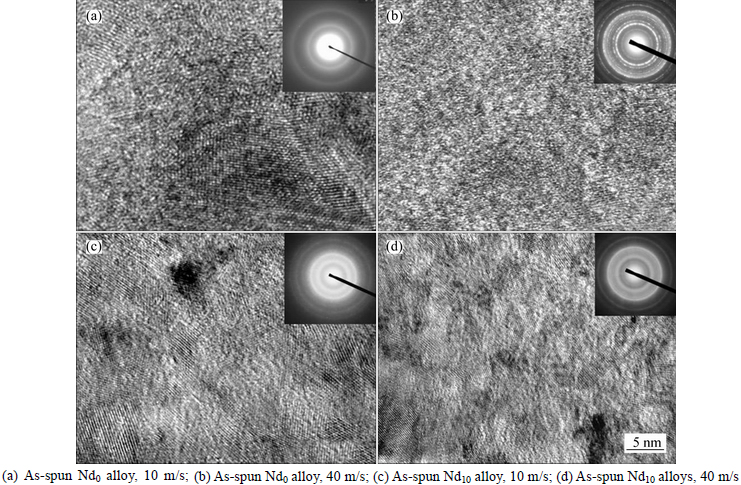
Fig. 2 HRTEM micrographs and ED patterns of as-spun (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys:

Fig. 3 SEM images (a, b, c, d) together with typical EDS spectra (e, f, g) of as-cast alloys:
3.2 Thermal stability and crystallization
To examine the thermal stability and the crystallization of the as-spun amorphous and nanocrystalline/amorphous alloys, DSC analysis was conducted, as demonstrated in Fig. 4. Evidently, some sharp exothermic DSC peaks emerge at about 460 °C, corresponding to a crystallization reaction (ordering) of the amorphous into nanocrystalline. Furthermore, we note that increasing the spinning rate from 0 to 40 m/s gives rise to the crystallization temperature rising from 452 to 483 °C, suggesting that rising spinning rate facilitates the stability of the amorphous structure.
3.3 Hydrogen storage properties
3.3.1 Hydrogen storage capacity and kinetics
Figure 5 describes the relationship between the hydrogen absorption quantities and the reaction time of the as-cast and spun Nd0 and Nd10 alloys at 200 °C. Evidently, all the as-cast and spun alloys display fast hydrogen absorption rates in the initial stage after that the hydrogen contents are almost saturated at the next quite a long hydrogenation time. For all the experimental alloys, the hydrogen absorption capacities in 100 min  are more than 95% of their saturated hydrogen absorption capacities. Thereby, it is considered to be reasonable taking
are more than 95% of their saturated hydrogen absorption capacities. Thereby, it is considered to be reasonable taking 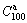 value as hydrogen absorption capacity of the alloy. Evidently, the as-spun alloys exhibit much higher
value as hydrogen absorption capacity of the alloy. Evidently, the as-spun alloys exhibit much higher 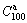 value than the as-cast ones, indicating that the melt spinning makes an obviously positive contribution to the hydrogen absorption capacity of the alloy. It is derived that enhancing the spinning rate from 0 to 40 m/s renders the
value than the as-cast ones, indicating that the melt spinning makes an obviously positive contribution to the hydrogen absorption capacity of the alloy. It is derived that enhancing the spinning rate from 0 to 40 m/s renders the 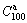 value growing from 2.95% to 3.58% for the Nd0 alloy and from 2.82% to 3.16% (30 m/s) and then to 3.01% for the Nd10 alloy.
value growing from 2.95% to 3.58% for the Nd0 alloy and from 2.82% to 3.16% (30 m/s) and then to 3.01% for the Nd10 alloy.

Fig. 4 DSC curves of as-spun Nd10 alloy

Fig. 5 Evolution of hydrogen absorption quantities of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with time varying at 200 °C:
As is wellknown, in addition to decreasing hydrogenation and dehydrogenation temperatures, improving the hydriding and dehydriding kinetics is also extremely important for putting Mg2Ni-type alloy into practice application. Therefore, it is very necessary to examine the influence of spinning rate on the hydriding and dehydriding kinetics of the alloy. Here, the hydriding kinetics of the alloy is characterized by its hydrogen absorption saturation ratio 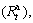 a ratio of the hydrogen absorption capacity at a fixed time to the saturated hydrogen absorption capacity of the alloy, which is defined as
a ratio of the hydrogen absorption capacity at a fixed time to the saturated hydrogen absorption capacity of the alloy, which is defined as 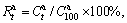 where
where  and
and 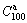 are hydrogen absorption capacities at t min and 100 min, respectively. Considering the better possibility of mutual comparison, we take the hydrogen absorption time of 5 min as a criterion and establish the relationship between the
are hydrogen absorption capacities at t min and 100 min, respectively. Considering the better possibility of mutual comparison, we take the hydrogen absorption time of 5 min as a criterion and establish the relationship between the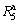 (t=5) values of the (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys and the spinning rate, just as provided in Fig. 6. Evidently, the growing of the spinning rate from 0 to 10 m/s results in the
(t=5) values of the (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys and the spinning rate, just as provided in Fig. 6. Evidently, the growing of the spinning rate from 0 to 10 m/s results in the 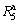 values of the alloys increasing dramatically, after that the growing rate of the
values of the alloys increasing dramatically, after that the growing rate of the 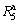 values clearly declines with the spinning rate rising, meaning that the melt spinning plays a beneficial role on the hydriding kinetics of the alloy. Particularly, the
values clearly declines with the spinning rate rising, meaning that the melt spinning plays a beneficial role on the hydriding kinetics of the alloy. Particularly, the 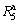 value is enhanced from 32.3% to 88.4% for the Nd0 alloy and from 36.9% to 91.5% for the Nd10 alloy by increasing the spinning rate from 0 to 40 m/s. Also, it is found that the minimum difference of the
value is enhanced from 32.3% to 88.4% for the Nd0 alloy and from 36.9% to 91.5% for the Nd10 alloy by increasing the spinning rate from 0 to 40 m/s. Also, it is found that the minimum difference of the 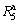 value between the as-spun and as-cast alloy for the fixed Nd content is much larger than the maximum difference of the
value between the as-spun and as-cast alloy for the fixed Nd content is much larger than the maximum difference of the 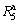 value generated by changing the Nd content for the fixed spinning rate. Hence, it can be surmised that the hydrogen absorption kinetics of the alloy is chiefly dominated by its structure.
value generated by changing the Nd content for the fixed spinning rate. Hence, it can be surmised that the hydrogen absorption kinetics of the alloy is chiefly dominated by its structure.

Fig. 6 Evolution of 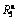 values of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with spinning rate varying
values of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with spinning rate varying

Fig. 7 Evolution of hydrogen desorption quantities of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with time varying at 250 °C:
Presented in Fig. 7 is the relationship between the hydrogen desorption quantities and the reaction time of the as-cast and spun (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys at 250 °C. An important feature of alloys emerging in the dehydriding process exhibits fast initial hydrogen desorption, subsequently the dehydrogenation rate dwindles sharply. Apparently, the melt spinning considerably enhances the dehydrogenation capability of the alloys. From Fig. 7, it is derived that the increasing of the spinning rate from 0 to 40 m/s engenders the  (H-desorbed capacity in 100 min) values growing from 0.30% to 1.98% for the Nd0 alloy and from 0.53% to 2.20% for the Nd10 alloy, respectively. Likewise, the hydrogen desorption kinetics of an alloy is symbolized by hydrogen desorption ratio
(H-desorbed capacity in 100 min) values growing from 0.30% to 1.98% for the Nd0 alloy and from 0.53% to 2.20% for the Nd10 alloy, respectively. Likewise, the hydrogen desorption kinetics of an alloy is symbolized by hydrogen desorption ratio  a ratio of the H-desorbed capacity at a fixed time to the saturated hydrogen absorption capacity of the alloy, which is defined as
a ratio of the H-desorbed capacity at a fixed time to the saturated hydrogen absorption capacity of the alloy, which is defined as  where
where 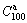 is the hydrogen absorption capacity at 100 min and
is the hydrogen absorption capacity at 100 min and  is the hydrogen desorption capacity at the time of t min, respectively. In order to facilitate comparison, here, we take hydrogen absorption time of 10 min as the standard. Thus, the relationship between the
is the hydrogen desorption capacity at the time of t min, respectively. In order to facilitate comparison, here, we take hydrogen absorption time of 10 min as the standard. Thus, the relationship between the  (t=10) values and the spinning rate of the (Mg24Ni10Cu2)100-xNdx (x= 0-20) alloys can be founded easily, as illustrated in Fig. 8. Evidently, the melt spinning makes a positive contribution to the dehydriding kinetics of the alloys. More specifically, enhancing the spinning rate from 0 to 40 m/s gives rise to the
(t=10) values and the spinning rate of the (Mg24Ni10Cu2)100-xNdx (x= 0-20) alloys can be founded easily, as illustrated in Fig. 8. Evidently, the melt spinning makes a positive contribution to the dehydriding kinetics of the alloys. More specifically, enhancing the spinning rate from 0 to 40 m/s gives rise to the  value rising from 8.2% to 38.9% for the Nd0 alloy and from 16.4% to 47.7% for the Nd10 alloy, respectively. What is more, it is noticeable that, whatever the spinning rate is, the Nd-added alloys show a much larger
value rising from 8.2% to 38.9% for the Nd0 alloy and from 16.4% to 47.7% for the Nd10 alloy, respectively. What is more, it is noticeable that, whatever the spinning rate is, the Nd-added alloys show a much larger  value than the as-cast one, suggesting that the addition of Nd facilitates the dehydriding rate of the alloy.
value than the as-cast one, suggesting that the addition of Nd facilitates the dehydriding rate of the alloy.
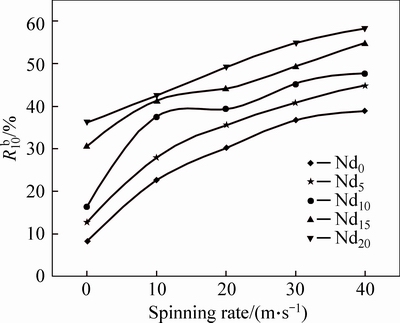
Fig. 8 Evolution of 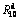 values of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with spinning rate varying
values of (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys with spinning rate varying
In the case of the improved hydriding and dehydriding kinetics by melt spinning, some elucidations can be provided. As known to all, the hydrogen absorption process of hydrogen storage alloys consists of the following steps: the rate of hydrogen molecular dissociation on the surface, the capability of hydrogen atoms penetrating from the oxide layer surface into the metal, the rate of hydrogen atoms diffusing into the bulk metal and through the hydride already formed, yet, the hydrogen absorption rate is predominated by the slowest step. The positive impact of the melt spinning on the hydriding kinetics is principally ascribed to the changed structure of the alloy by the melt spinning. The crystalline material, when melt spun, becomes at least partially disordered and its structure turns into nanocrystalline, generating a lot of new crystallites and grain boundaries (Fig. 2). Hence, some crystal defects such as dislocations, stacking faults and grain boundaries are introduced, as evidenced by our previous work [22], which may promote the diffusion of hydrogen in materials by providing numerous sites with low diffusion activation energy [23]. Moreover, it has been ascertained that the hydriding and dehydriding kinetics of the Mg2Ni-type alloy is very sensitive to its structure. Especially, the hydrogen storage properties are considered to be strongly affected by their nanometer scale structures because of the thermodynamic and kinetic aspects. As stated by KUMAR et al [24], the changing of the structure of the Mg2Ni alloy from polycrystalline to nanocrystalline results in a drop of about 100 K in absorbing/desorbing temperature, namely from 573 K to 473 K. It is evidenced by ZHAO et al [25] that hydrogen adheres to the surface of the nanocrystalline nickel more strongly than that of the polycrystalline nickel, facilitating the dissociation reaction of hydrogen molecular. With respect to the positive contribution of Nd adding to the dehydriding kinetics, it is considered to be related to the following two reasons [26]. Firstly, the addition of Nd facilitates the glass forming of Mg2Ni-type alloy, and amorphous Mg2Ni shows an excellent hydrogen desorption capability. Secondly, such addition decreases the stability of the hydride and makes the desorption reaction easier.
3.3.2 Hydriding and dehydriding cycle stability
Cycle stability, one of the major performances which are used to evaluate whether a kind of an alloy can be applied as a hydrogen storage material or not, is signified by the capacity retaining rate (Sn), defined as  where
where 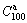 is the saturated hydrogen capacity and Cn is the hydrogen absorption capacity at the nth hydriding and dehydriding cycle, respectively. Evidently, it means that the larger the capacity retaining rate (Sn) is, the better the cycle stability of the alloy will be. The variations of the Sn values of the as-spun (40 m/s) Nd0 and Nd10 alloys depending on the cycle numbers are provided in Fig. 9, from which the degradation process of the hydrogen capacity of the alloys can be seen clearly. In order to directly exhibit the degradation of the hydrogen capacity with the cycle number, the evolution of the
is the saturated hydrogen capacity and Cn is the hydrogen absorption capacity at the nth hydriding and dehydriding cycle, respectively. Evidently, it means that the larger the capacity retaining rate (Sn) is, the better the cycle stability of the alloy will be. The variations of the Sn values of the as-spun (40 m/s) Nd0 and Nd10 alloys depending on the cycle numbers are provided in Fig. 9, from which the degradation process of the hydrogen capacity of the alloys can be seen clearly. In order to directly exhibit the degradation of the hydrogen capacity with the cycle number, the evolution of the 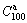 values of the Nd0 and Nd10 alloys with cycle number is also inserted in Fig. 9. The slopes of the curves qualitatively reflect the degradation rate of the hydrogen capacity during the hydriding and dehydriding cycles, namely the smaller the slope of the curve is, the better the cycle stability of the alloy will be. It can be seen that the Sn values of the as-spun (40 m/s) Nd0 and Nd10 alloys exhibit small drop with cycle number increasing, reflecting excellent cycle stability. Stated concretely, after cycling for 100 times, the Sn values of the Nd0 and Nd10 alloys still keep at 88.3% and 96.8%, respectively. Noticeably, the slope of the as-spun Nd10 alloy is less than that of the Nd0 one, indicating that the addition of Nd plays a beneficial role on the cycle stability of the alloy.
values of the Nd0 and Nd10 alloys with cycle number is also inserted in Fig. 9. The slopes of the curves qualitatively reflect the degradation rate of the hydrogen capacity during the hydriding and dehydriding cycles, namely the smaller the slope of the curve is, the better the cycle stability of the alloy will be. It can be seen that the Sn values of the as-spun (40 m/s) Nd0 and Nd10 alloys exhibit small drop with cycle number increasing, reflecting excellent cycle stability. Stated concretely, after cycling for 100 times, the Sn values of the Nd0 and Nd10 alloys still keep at 88.3% and 96.8%, respectively. Noticeably, the slope of the as-spun Nd10 alloy is less than that of the Nd0 one, indicating that the addition of Nd plays a beneficial role on the cycle stability of the alloy.

Fig. 9 Evolution of capacity maintaining rate (Sn) together with hydrogen absorption capacity of as-spun (40 m/s) Nd0 and Nd10 alloys with cycle number
In an attempt to elucidate the possible reasons for the cycle performance loss of the as-spun alloys, we investigate the structure changes of the alloy generated by hydriding and dehydriding cycle. First, the morphologies of the Nd0 and Nd10 alloy particles before and after cycling are observed by SEM, as illustrated in Fig. 10. A lot of cracks can be easily seen on the surfaces of the alloy particles after 20 cycles, and with cycle number increasing, the pulverization degree of the particles becomes more serious. Noticeably, we find that there are much fewer cracks on the surface of the Nd10 alloy particle after cycling than that of the Nd0 one, reflecting that the addition of Nd can clearly enhance the anti-pulverization ability of the alloy. The faster capacity degradation of the as-spun (40 m/s) Nd0 alloy is principally ascribed to the structure stability of Nd0 alloy which is lower than Nd10 alloy. The crystal defects of Nd0 alloy prone to merge even disappear during the hydriding-dehydriding cycle, which results in the occupied site of atomic hydrogen losing and the capacity decreasing. The function of Nd adding is surmised to be ascribed to two aspects. On the one hand, adding the rare elements (La, Nd, Sm and Y) engenders the cell volume of the Mg2Ni alloy enlarging clearly [18], decreasing the ratios of expansion/contraction in the process of hydrogen absorption/desorption, hence increasing the anti-pulverization capability. On the other hand, the glass formation facilitated by Nd adding is extremely important because an amorphous phase improves not only anti-pulverization ability but also anti-corrosion and anti-oxidation abilities of the alloy electrode in a corrosive electrolyte [19, 27]. It must be pointed out that the capacity degradation of the alloys can not be ascribed to the pulverization, which is supported by two aspects. Firstly, the cycle testing is carried out under almost absolute vacuum, which prevents the surfaces of alloy particles from being oxidized. Secondly, it is generally accepted that the reduction of the alloy particles’ size facilitates to enhance hydrogen storage capacity and kinetics of the alloys. What is more, we investigate the crystallization behavior of amorphous structure. As considered by LIANG et al [14], the crystallization of amorphous phase in the Mg-based alloy causes an obvious change of hydrogen storage performances. As a matter of fact, the result shown in Fig. 4 indicates that crystallization of the amorphous phase will not occur at the dehydrogenation temperature because the crystallization temperatures of the amorphous phase in the alloys are all above 452 °C, much higher than dehydrogenation temperature (250 °C). But in fact, the crystallization of the amorphous phase is inevitable during repeated cycling in spite of the crystallization temperature being much higher than the dehydrogenation one, which is also validated by XRD data, as depicted in Fig. 11. Obviously, the as-spun Nd10 alloy still holds amorphous structure but only a little crystal Mg2Ni phase appears after 20 cycles. After cycling for 50 times, the amorphous structure is found to turn into nanocrystalline completely. Based on the above-mentioned results, it can be concluded that the degradation of cycling properties of the as-spun alloy is principally ascribed to the varying structure, particularly crystallization of amorphous phase.

Fig. 10 SEM images of granular morphologies of as-spun (40 m/s) Nd0 and Nd10 alloys before and after hydriding and dehydriding cycles:

Fig. 11 XRD patterns of as-spun (40 m/s) Nd10 alloy before and after hydriding and dehydriding cycle
4 Conclusions
The hydrogen storage characteristics of the as-cast and spun (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys and their structure evolution during cycling were investigated. The major conclusions are summarized as follows:
1) All the as-cast alloys hold a multiphase structure, consisting of the major phase Mg2Ni and some secondary phases. The addition of Nd renders Nd5Mg41 and NdNi phases forming whose amounts markedly grow with the Nd content increasing. The addition of Nd facilitates the glass forming in the Mg2Ni-type alloy and the degree of amorphization significantly increases with the Nd content rising.
2) The melt spinning exerts a significantly positive impact on the hydrogen absorption capacity together with hydriding and dehydriding kinetics of the alloys, which is principally attributed to the nanocrystalline and amorphous structure generated by melt spinning.
3) The as-spun alloys display excellent cycle stability, to be attributed to high stability of the nanocrystalline and amorphous structure generated by melt spinning. The repeated cycling for many times results in a gradual evolution of the nanocrystalline and amorphous structure, namely the crystallization of amorphous, which is responsible for the degradation of the cycle performances.
References
[1] LIU Yong-feng, PAN Hong-ge, GAO Ming-xia, WANG Qi-dong. Advanced hydrogen storage alloys for Ni/MH rechargeable batteries [J]. J Mater Chem, 2011, 21(13): 4743-4755.
[2] SAKINTUNA B, LAMARI-DARKRIM F, HIRSCHER M. Metal hydride materials for solid hydrogen storage: A review [J]. International Journal of Hydrogen Energy, 2007, 32(9): 1121-1140.
[3] HUANG Tai-zhong, WU Zhu, XIA Bao-jia, CHEN Jin-zhou, YU Xue-bin, XU Nai-xin, LU Chang-wei, YU Hui-mei. TiCr1.2(V-Fe)0.6—A novel hydrogen storage alloy with high capacity [J]. Science and Technology of Advanced Materials, 2003, 4(6): 491-494.
[4] SCHLAPBACH L,  A. Hydrogen-storage materials for mobile applications [J]. Nature, 2001, 414: 353-358.
A. Hydrogen-storage materials for mobile applications [J]. Nature, 2001, 414: 353-358.
[5] EBRAHIMI-PURKANI A, KASHANI-BOZORG S F. Nanocrystalline Mg2Ni-based powders produced by high-energy ball milling and subsequent annealing [J]. Journal of Alloys and Compounds, 2008, 456(1-2): 211-215.
[6] CHANDRA D, SHARMA A, CHELLAPPA R. Hydriding and structural characteristics of thermally cycled and cold-worked V–0.5%C alloy [J]. Journal of Alloys and Compounds, 2008, 452(2): 312-324.
[7] XIE D H, LI P, ZENG C X. Effect of substitution of Nd for Mg on the hydrogen storage properties of Mg2Ni alloy [J]. Journal of Alloys and Compounds, 2009, 478(1/2): 96-102.
[8] ZHANG Yang-huan, ZHAO Dong-liang, LI Bao-wei, MA Zhi-hong, GUO Shi-hai, WANG Xin-lin. Influence of substituting Ni with Co on hydriding and dehydriding kinetics of melt spun nanocrystalline and amorphous Mg2Ni-type alloys [J]. Journal of Central South University of Technology, 2011, 18(2): 303-309.
[9] ZHANG Yang-huan, YANG Tai, BU Wen-gang, CAI Ying, ZHANG Guo-fang, ZHAO Dong-liang. Effect of Nd content on electrochemical performances of nanocrystalline and amorphous (Mg24Ni10Cu2)100-xNdx (x=0-20) alloys prepared by melt spinning [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(12): 3668-3676.
[10] ZHANG Yang-huan, XU Sheng, ZHAI Ting-ting, YANG Tai, YUAN Ze-ming, ZHAO Dong-liang. Hydrogen storage kinetics of nanocrystalline and amorphous Cu-Nd-added Mg2Ni-type alloys [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3524-3533.
[11] ZHANG Yang-huan, YANG Tai, ZHAI Ting-ting, SHANG Hong-wei, ZHANG Guo-fang, ZHAO Dong-liang. Influences of substituting Ni with M (M=Cu, Co, Mn) on gaseous and electrochemical hydrogen storage kinetics of Mg20Ni10 alloys [J]. Journal of Central South University, 2014, 21(5): 1705-1713.
[12] WU Mao-sung, WU Hong-rong, WANG Yung-yun, WAN Chi-chao. Surface treatment for hydrogen storage alloy of nickel/metal hydride battery [J]. Journal of Alloys and Compounds, 2000, 302(1/2): 248-257.
[13] ZHANG Yang-huan, HAN Zhong-gang, YUAN Ze-ming, YANG Tai, QI Yan, ZHAO Dong-liang. Electrochemical properties of nanocrystalline and amorphous Mg–Y–Ni alloys applied to Ni-MH battery [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(11): 3736-3746.
[14] LIANG G Y, WU D C, LI L, HUANG L J. A discussion on decay of discharge capacity for amorphous Mg–Ni–Nd hydrogen storage alloy [J]. Journal of Power Sources, 2009, 186(2): 528-531.
[15] ZHANG Yang-huan, ZHANG Guo-fang, LI Xia, HOU Zhong-hui, REN Hui-ping, ZHAO Dong-liang. Structure and hydrogen storage kinetics of asspun Mg2Ni type alloys [J]. Journal of Central South University: Science and Technology, 2012, 43(6): 2101-2107. (in Chinese)
[16] TODOROVA S, SPASSOV T. Mg6Ni formation in rapidly quenched amorphous Mg–Ni alloys [J]. Journal of Alloys and Compounds, 2009, 469(1/2): 193-196.
[17] ZHANG Yang-huan, SONG Chun-hong, REN Hui-ping, LI Zhi-gang, HU Feng, ZHAO Dong-liang. Enhanced hydrogen storage kinetics of nanocrystalline and amorphous Mg2Ni-type alloy by substituting Ni with Co [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(9): 2002-2009.
[18] ZHANG Yang-huan, LI Bao-wei, REN Hui-ping, HOU Zhong-hui, HU Feng, WANG Xin-lin. Influences of melt spinning on electrochemical hydrogen storage performance of nanocrystalline and amorphous Mg2Ni-type alloys [J]. Journal of Central South University of Technology, 2011, 18(6): 1825-1832.
[19] ZHANG Yang-huan, QI Yan, REN Hui-ping, MA Zhi-hong, GUO Shi-hai, ZHAO Dong-liang. Hydriding and dehydriding kinetics of nanocrystalline and amorphous Mg2Ni1-xMnx (x=0-0.4) alloys prepared by melt spinning [J]. Journal of Central South University of Technology, 2011, 18(4): 985-992.
[20] ZHANG Yang-huan, ZHAO Dong-liang, LI Bao-wei, QI Yan, GUO Shi-hai, WANG Xin-lin. Hydrogen storage behaviours of nanocrystalline and amorphous Mg20Ni10-xCox (x=0-4) alloys prepared by melt spinning [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(3): 405-411.
[21] ZHENG Cai-xing, LIU Rang-su, PENG Ping, ZHOU Qun-yi. Simulation study for atomic size and alloying effects during forming processes of amorphous alloys. [J] Science in China Series G: Physics, Mechanics & Astronomy, 2004, 47(4): 393-402.
[22] ZHANG Yang-huan, LI Bao-wei, REN Hui-ping, HU Feng, ZHANG Guo-fang, GUO Shi-hai. Gaseous and electrochemical hydrogen storage kinetics of nanocrystalline Mg2Ni-type alloy prepared by rapid quenching [J]. Journal of Alloys and Compounds, 2011, 509(18): 5604-5610.
[23] WU Y, HAN W, ZHOU S X, LOTOTSKY M V, SOLBERG J K, YARTYS V A. Microstructure and hydrogenation behavior of ball-milled and melt-spun Mg-10Ni-2Mm alloys [J]. Journal of Alloys and Compounds, 2008, 466(1/2): 176-181.
[24] KUMARA L H, VISWANATHAN B, MURTHY S S. Hydrogen absorption by Mg2Ni prepared by polyol reduction [J]. Journal of Alloys and Compounds, 2008, 461(1/2): 72-76.
[25] ZHAO Xiang-yu, DING Yi, MA Li-qun, WANG Lin-ying, YANG Meng, SHEN Xiao-dong. Electrochemical properties of MmNi3.8Co0.75Mn0.4Al0.2 hydrogen storage alloy modified with nanocrystalline nickel [J]. International Journal of Hydrogen Energy, 2008, 33(22): 6727-6733.
[26] LASS E A. Hydrogen storage measurements in novel Mg-based nanostructured alloys produced via rapid solidification and devitrification [J]. International Journal of Hydrogen Energy, 2011, 36(17): 10787-10796.
[27] ZHANG Yang-huan,  Ke, ZHAO Dong-liang, GUO Shi-hai, QI Yan, WANG Xin-lin. Electrochemical hydrogen storage characteristics of nanocrystalline and amorphous Mg2Ni-type alloys prepared by melt-spinning [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 502-511.
Ke, ZHAO Dong-liang, GUO Shi-hai, QI Yan, WANG Xin-lin. Electrochemical hydrogen storage characteristics of nanocrystalline and amorphous Mg2Ni-type alloys prepared by melt-spinning [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 502-511.
(Edited by FANG Jing-hua)
Foundation item: Projects(51161015, 51371094) supported by the National Natural Science Foundation of China
Received date: 2015-10-16; Accepted date: 2015-12-04
Corresponding author: ZHANG Yang-huan, Professor, PhD; Tel: +86-10-62183115; E-mail: zhangyh59@sina.com
Abstract: Nanocrystalline and amorphous Mg-Nd-Ni-Cu quaternary alloys with a composition of (Mg24Ni10Cu2)100-xNdx (x=0, 5, 10, 15, 20) were prepared by melt spinning technology and their structures as well as gaseous hydrogen storage characteristics were investigated. The XRD, TEM and SEM linked with EDS detections reveal that the as-spun Nd-free alloy holds an entire nanocrystalline structure but a nanocrystalline and amorphous structure for the as-spun Nd-added alloy, implying that the addition of Nd facilitates the glass forming in the Mg2Ni-type alloy. Furthermore, the degree of amorphization of the as-spun Nd-added alloy and thermal stability of the amorphous structure clearly increase with the spinning rate rising. The melt spinning ameliorates the hydriding and dehydriding kinetics of the alloys dramatically. Specially, the rising of the spinning rate from 0 (the as-cast was defined as the spinning rate of 0 m/s) to 40 m/s brings on the hydrogen absorption saturation ratio 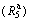 (a ratio of the hydrogen absorption quantity in 5 min to the saturated hydrogen absorption capacity) increasing from 36.9% to 91.5% and the hydrogen desorption ratio
(a ratio of the hydrogen absorption quantity in 5 min to the saturated hydrogen absorption capacity) increasing from 36.9% to 91.5% and the hydrogen desorption ratio 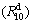 (a ratio of the hydrogen desorption quantity in 10 min to the saturated hydrogen absorption capacity) rising from 16.4% to 47.7% for the (x=10) alloy, respectively.
(a ratio of the hydrogen desorption quantity in 10 min to the saturated hydrogen absorption capacity) rising from 16.4% to 47.7% for the (x=10) alloy, respectively.
- Effect of melt spinning on gaseous hydrogen storage characteristics of nanocrystalline and amorphous Nd-added Mg2Ni-type alloys


