
Preparation of bismuth oxide/titania composite particles and their photocatalytic activity to degradation of 4-chlorophenol
XU Jing-jing1, 2, CHEN Min-dong1, FU De-gang3
1. Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control,College of Environmental Science and Engineering, Nanjing University of Information Science and Technology, Nanjing 210044, China;
2. State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering,Hohai University, Nanjing 210098, China
3. State Key Laboratory of Bioelectronics, Southeast University, Nanjing 210096, China
Received 22 October 2009; accepted 27 May 2010
Abstract:
Bismuth oxide/titania, one interfacial composite semiconductor with high photocatalytic activity under solar light, was prepared at low temperature. The structure was characterized by X-ray diffractometry (XRD), scanning electron microscopy (SEM), brunauer-emmett-teller (BET), X-ray photoelectron spectroscopy (XPS) and diffuse reflection spectra (DRS). The results indicate that deposited titania nanoparticles on bismuth oxide surface have micro-nano structure, and this composite material exhibits porosity and increased surface hydroxyl groups. Furthermore, the as-prepared photocatalyst shows higher photocatalytic activity to the degradation of 4-chlorophenol than pure titania or P25 under sunlight.
Key words:
bismuth oxide; titania; photocatalysis; 4-chlorophenol;
1 Introduction
As a promising material, titania (TiO2) has been widely used in the photocatalytic degradation of polluted water and air[1-3]. However, the fast recombination rate of photo-generated charge carriers hinders the commercialization of this technology[1]. The energy gap between the valence band (VB) and conduction band (CB) in pure TiO2 is 3.2eV, so UV light is necessary to excite electrons on the TiO2 surface. To activate the photocatalysts with higher efficiency and longer wavelength, a number of strategies have been adopted. One of those strategies is to couple TiO2 with other semiconductor with appropriate CB and VB gaps. Photocatalysts SiO2-TiO2[4], CdS-TiO2[5], ZnO-TiO2[6] and SnO2-TiO2[7] have been prepared and used for many reactions.
It is well known that the lifetime of photo-induced charge carries is a key factor for improving photocatalytic activity, and using appropriate composite TiO2 can accelerate the separation of electrons and holes. As for ZnO-TiO2[8], the electrons transfer from the CB of ZnO to that of TiO2 under illumination, and conversely, the holes transfer from the VB of TiO2 to that of ZnO. Thus, the lifetime of photo-induced pairs increased since their recombination rate decreased. In order to extend the range of excitation energies of TiO2 into visible region, materials of narrow band gap were coupled with TiO2. SHINGUU et al[9] reported that incorporated WO3 with TiO2 layers could modify the grain size and surface, and more important, it showed enhanced photocatalytic activity under visible light irradiation.
Bismuth oxide (Bi2O3) is used in a variety of areas, such as sensor technology, optical coatings and electrochromic materials[10-12], due to its high refractive index, dielectric permittivity, marked photoconductivity and photoluminescence[10]. These special features explain the great effort devoted to the investigation of Bi2O3 polymorphs over the past few years. Bi2O3 has five main polymorphic forms, denoted by α-, β-, γ-, δ- and ω-Bi2O3[13-14]. Among them, the band gaps of the low-temperature α-phase and high-temperature metastable β-phase are 2.85 eV and 2.58 eV, respectively[15]. Thus, Bi/TiO2 may exhibit excellent photocatalytic activity. KANG et al[16] found that without H2O addition, Bi/TiO2 exhibited higher photocatalytic activity than TiO2 in decomposing CH3CHO. However, HONG et al[17] reported that whether adding H2O or not, the activity of photocatalytic degradation of benzene by Bi/TiO2 was lower than that of pure TiO2 under UV irradiation. Among those investigations, the activity of Bi2O3/TiO2 compound particles under solar light has not been concerned.
In this work, Bi2O3/TiO2 composite particles were synthesized by sol-gel method under mild condition. The characterization of the as-prepared sample was measured by XRD, SEM, XPS, BET and DRS. The photocatalytic activity was evaluated under solar light irradiation for degradation of 4-chlorophenol.
2 Experimental
2.1 Catalyst preparation
Bi2O3 powders were purchased from Sinopham Chemical Reagent Co., Ltd, with a size range of 0.5-2 μm and mainly in monoclinic phase. Titanium (IV)-n-butoxide (Ti(OBu)4) was used as Ti precursor. Bi2O3 was firstly added into abundant water, the pH value of which was adjusted to 2.0 by HNO3. Then the mixture of Ti(OBu)4 and isopropyl alcohol was added dropwise into the solution. After complete hydrolysis of Ti(OBu)4, the solution was refluxed at 70 °C for 20 h and a sol solution formed. Finally, the as-prepared sol was vacuum dried at 60 °C for 2 h to obtain Bi2O3/TiO2 composite powders. For comparison, pure TiO2 powders were also synthesized using the method mentioned above without adding Bi2O3.
2.2 Characterization
The as-prepared samples were identified by X-ray diffractometer (XRD, XD-3A, Shimadazu Corporation, Japan) using graphite monochromatic copper radiation (Cu Kα) at 40 kV, 30 mA over the 2θ range of 20°-80°. The morphologies were characterized with a scanning electron microscope (SEM, Sirion, FEI). BET surface area measurements were carried out by N2 adsorption at 77 K using an ASAP2020 instrument. The total pore volume was calculated from the amount of nitrogen adsorbed at relative pressure of 0.975. The binding energy was identified by X-ray photoelectron spectroscope (XPS) with Mg Kα radiation (ESCALB- 250). A UV-vis spectrophotometer (Shimadzu UV-4100) was used to record the diffuse reflectance spectra of samples.
2.3 Photocatalytic degradation experiments
The photocatalytic activity of Bi2O3/TiO2 particles was investigated by degradation of 4-chlorophenol in the aqueous solution. The bench-scale photoreactor system was composed of a cylindrical silica reactor, and a light filter cutting light with wavelength shorter than 400 nm providing artificial solar light from vertical irradiation. A set of photocatalytic degradation experiments were performed with the following procedure: 200 mg TiO2 powder was added into 200 mL 4-chlorophenol solution of 50 mg/L. The suspension was stirred in dark for 30 min to obtain adsorption equilibrium of 4-chlorophenol before illumination. At a defined time interval, 5 mL suspension was removed and the concentration of 4-chlorophenol was analyzed using the UV-vis spectrophotometer at 280 nm.
3 Results and discussion
3.1 XRD analysis
The X-ray diffraction (XRD) patterns of Bi2O3/TiO2, pure TiO2 and Bi2O3 powders are shown in Fig.1. It can be seen that two crystalline phases are identified from the XRD patterns of TiO2, namely the major phase is anatase, and the minor phase is brookite. Bi2O3 is mainly in monoclinic phase. As for Bi2O3/TiO2, the peak at 25.4° is broadened, indicating that there exist more than one peak, which may contain anatase and α-bismuth oxide peaks compared with those of Bi2O3 and pure TiO2. No new phase is detected, which indicates that the TiO2 particles were only adsorbed on the surface of Bi2O3.
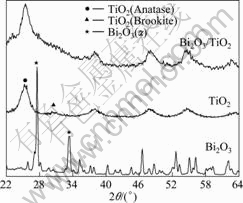
Fig.1 XRD patterns of samples
3.2 Microstructure
In order to probe the interaction among the components in the composite photocatalysts, these photocatalysts were investigated. The SEM image of Bi2O3/TiO2 sample is shown in Fig.2. A big particle with regular monoclinic structure in micrometer size is α-Bi2O3, and the clusters deposited on the surface of Bi2O3 are TiO2 nanoparticles. It can be seen that the composite sample is composed of micro-scale Bi2O3 particles and sub-micro-scale clusters containing titania nano-particles. This micro-nano structure with

Fig.2 SEM image of Bi2O3/TiO2 particles
hierarchical structure containing micro, sub-micro and nano-scale particles may be beneficial for the achievement of various photo-electric properties[18]. On the other hand, the dispersity of titania is enhanced. Therefore, this structure can offer more active adsorption sites and photocatalytic reaction centers.
3.3 BET
Fig.3 shows nitrogen adsorption-desorption isotherms of TiO2 and Bi2O3/TiO2. Both samples show the isotherm of type IV (BDDT classification)[19]. At high relative pressure from 0.4 to 0.8, the isotherms of TiO2 and Bi2O3/TiO2 exhibit hysteresis loops of type H2 and H3, respectively, this indicates that the powders contained mesopores (2-50 nm).

Fig.3 Adsorption-desorption isotherms of TiO2 (a) and Bi2O3/TiO2 (b)
Fig.4 shows the pore diameter distribution of Bi2O3/TiO2. It can be seen that the diameter range of pore mainly distributes from 2.0 to 9.0 nm, and the average pore diameter is 5.0 nm, which is larger than the value of 3.2 nm for TiO2. The formation of mesoporous structure in TiO2 and Bi2O3/TiO2 is attributed to the aggregation of TiO2 particles[20-21]. As seen from Table 1, the BET surface area of the composite Bi2O3/ TiO2 is smaller than that of pure titania due to the bigger nonporous particles of Bi2O3. When the pore volume becomes larger, bigger crystallites aggregate to bigger pores[21].
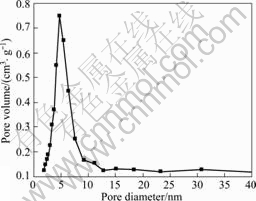
Fig.4 Pore diameter distribution of Bi2O3/TiO2
Table 1 BET data for TiO2 and Bi2O3/TiO2

3.4 XPS
In order to analyze the chemical composition and purity of the prepared samples, the XPS spectrum of Bi2O3/TiO2 was measured. The high-resolution XPS spectra of Bi 4f, Ti 2p and O 1s regions on the surface of samples are shown in Fig.5. The peaks of Bi 4f 7/2 and Bi 4f 5/2 are centered at 164.2 and 158.9 eV, respectively, which are consistent with those of Bi3+, as reported in Ref.[17]. This XPS spectrum demonstrates that the main valence of Bi in the prepared sample is +3 in Bi2O3. Ti 2p 1/2 and Ti 2p 3/2 spin-orbital splitting photoelectrons were located at the binding energies of about 464.3 and 458.2 eV, respectively. In general, large binding energy means more oxidized metal[22]. However, no evidence indicated that the band shifted to higher binding energy when Bi2O3 was incorporated with TiO2, which was also confirmed by KANG et al[16]. As shown in Fig. 5(c), the O 1s spectra of both TiO2 and Bi2O3/TiO2 samples are fitted to two peaks, respectively. The lower binding energy of 529.8 eV is attributed to Ti—O in TiO2 crystal

Fig.5 XPS patterns of Bi2O3/TiO2 composite samples: (a) Bi 4f, (b) Ti 2p; (c) O 1s core level
lattice, and the higher binding energy of 531.4 eV is related to O—H resulting from chemisorbed water[23]. The amount ratio of O—H/Ti—O for Bi2O3/TiO2 increases compared with pure TiO2. The ratios are 0.41 and 0.21, respectively. It illuminates that the number of surface hydroxyl groups of TiO2 is increased when incorporated with Bi2O3, and subsequently free hydroxyl radicals which was proved to be beneficial for photocatalytic reactions[24] can be also increased.
3.5 DRS
The UV-vis diffuse reflectance spectra of TiO2 and Bi2O3/TiO2 are shown in Fig.6. It can be seen that there is a significant shift in the onset absorption towards the higher wavelength of Bi2O3/TiO2. As known from XRD pattern, no new phase appears in Bi2O3/TiO2, indicating that it is not Bi12TiO20 with band gap of 2.4 eV[25] or other phase leads to red shift, but the band gap of α-Bi2O3 of 2.85 eV responds to visible irradiation.

Fig.6 UV-vis diffuse reflectance spectra of TiO2 and Bi2O3/TiO2
3.6 Photocatalytic activity
The photocatalytic activity of TiO2 and Bi2O3/TiO2 particles were studied by decomposition of 4-chlorophenol aqueous solution under solar light illumination. It can be seen from Fig.7 that Bi2O3/TiO2 exhibits much higher photocatalytic activity than TiO2 or P25. The degradation rates of Bi2O3/TiO2, TiO2 and P25 are 78.6%, 20.8% and 6.1%, respectively, which is in good agreement with analyses forenamed. It is well known that the photocatalytic oxidation of organic pollutants in aqueous suspension follows Langmuir-Hinshelwood model:
![]() (1)
(1)
where (-dc/dt) is the degradation rate of 4-chlorophenol; c is the 4-chlorophenol concentration in the solution; t is reaction time; kr is the reaction rate constant; and Ka is the adsorption coefficient of the reactant. Kac is negligible when the value of c is very small. As a result, Eq.(1) can be described as a first-order kinetics. Set Eq.(1) under the initial conditions of the photocatalytic procedure, when t=0, c=c0, it can be described as
![]() (2)
(2)
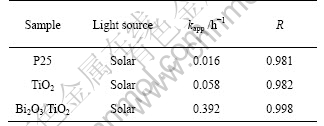
where kapp is the apparent rate constant, used as the basic kinetic parameter for different photocatalysts, and it can determine the photocatalytic activity independent of the previous adsorption period in the dark and the concentration of 4-chlorophenol remaining in the solution[26]. The variations in ln(c0/c) as a function of irradiation time are given in Fig.8, and the corresponding kapp and R (regression relative coefficient) are given in Table 2, which confirms that kapp is enhanced by incorporating TiO2 with Bi2O3, under solar illumination compared wtih pure one.
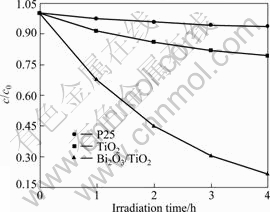
Fig.7 Kinetic of 4-chlorophenol degradation with different samples

Fig.8 Variations in ln (c0/c) as function of irradiation time and linear fits of samples
Table 2 kapp and R data for each sample
4 Conclusions
1) The as-prepared material does not form new crystal phase besides anatase from TiO2 and monoclinic Bi2O3 phase. Depositing TiO2 nanoparticles on Bi2O3 surface can form micro-nano structure.
2) Bi2O3/TiO2 composite particles exhibit porosity and increased surface hydroxyl groups. Furthermore, more active adsorption sites and photocatalytic reaction centers can be offered due to their special structure.
3) Owing to the narrow band gap of α-Bi2O3, Bi2O3/TiO2 shows red shift compared with pure TiO2. The photocatalytic activity of Bi2O3/TiO2 has been enhanced a lot compared with P25 and pure TiO2 under sunlight.
References
[1] HOFFMANN M R, MARTIN S T, CHOI W, BAHNEMANN D W. Environmental applications of semiconductor photocatalysis [J]. Chem Rev, 1995, 95(1): 69-96.
[2] WANG Chao, AO Yan-hui, WANG Pei-fang, HOU Jun, QIAN Jin, ZHANG Song-he. Preparation, characterization, photocatalytic properties of titania hollow sphere doped with cerium [J]. J Hazard Mater, 2010, 178(1-3): 517-521.
[3] WANG Chao, AO Yan-hui, WANG Pei-fang, HOU Jun, QIAN Jin. Photocatalytic performance of Gd ion modified titania porous hollow spheres under visible light [J]. Mater Letts, 2010, 64(8): 1003-1006.
[4] ARAI Y, TANAKA K, KHLAIFAT A L. Photocatalysis of SiO2-loaded TiO2 [J]. J Mole Catal A, 2006, 243(1): 85-88.
[5] BISWAS S, HOSSAIN M F, TAKAHASHI T, KUBOTA Y, FUJISHIMA A. Photocatalytic activity of high-vacuum annealed CdS–TiO2 thin film [J]. Thin Solid Film, 2008, 516(21): 7313-7317.
[6] HOU?KOV? V, ?TENGL V, BAKARDJIEVA S, MURAFA N. Photoactive materials prepared by homogeneous hydrolysis with thioacetamide: Part 2—TiO2/ZnO nanocomposites [J]. J Phys Chem Solid, 2008, 69(7): 1623-1631.
[7] LEE H C, HWANG W S. Substrate effects on the oxygen gas sensing properties of SnO2/TiO2 thin films [J]. Appl Surf Sci, 2006, 253(4): 1889-1897.
[8] SERPONE N, PICHAT P, PILIZZETTI E, HIDAKA H. Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: chemical evidence for electron and hole transfer between coupled semiconductors [J]. J Photochem Photobio A, 1995, 85(3): 247-255.
[9] SHINGUU H, BHUIYAN M M H, IKEGAMI T, EBIHARA K. Preparation of TiO2/WO3 multilayer thin film by PLD method and its catalytic response to visible light [J]. Thin Solid Films, 2006, 506-507: 111-114.
[10] LEONTIE L, CARAMAN M, ALEXE M, HARNAGEA C. Structural and optical characteristics of bismuth oxide thin films [J]. Surf Sci, 2002, 507-510: 480-485.
[11] TOMCHENKO A A. Structure and gas-sensitive properties of WO3-Bi2O3 mixed thick Films [J]. Sensor Actuat B, 2000, 68(1-3): 48-52.
[12] SHIMIZUGAWA K, SUGIMOTO M, MIURA N, YAMAZOE N. Bismuth oxide thin film as new electrochromic material [J]. Solid State Ionics, 1998, 113-115: 415-419.
[13] MONNEREAU O, TORTET L, LLEWELLYN P, ROUQUEROL F, VACQUIER G. Synthesis of Bi2O3 by controlled transformation rate thermal analysis: A new route for this oxide [J]. Solid State Ionics 2003, 157(1-4): 163-169.
[14] GUALTIERI A F, IMMOVILLI S, PRUDENZIATI M. Reduction process of RuO2 powder and kinetics of compound omega-Bi2O3 [J]. Powder Diffract, 1997, 12(2): 90-92.
[15] DOLOCAN V. Transmission spectra of bismuth trioxide thin films [J]. Appl Phys, 1978, 16: 405-407.
[16] KANG M, KO Y R, JEON M K, LEE S C, CHOUNG S J, PARK J Y, KIM S, CHOI S H. Characterization of Bi/TiO2 nanometer sized particle synthesized by solvothermal method and CH3CHO decomposition in a plasma-photocatalytic system [J]. J Photochem Photobio A, 2005, 173(2): 128-136.
[17] HONG W J, KANG M. The super-hydrophilicities of Bi-TiO2, V-TiO2, and Bi-V-TiO2 nano-sized particles and their benzene photodecompositions with H2O addition [J]. Mater Lett, 2006, 60(9-10): 1296-1305.
[18] ZHAO Yao, ZHAI Jin, TAN Shu-xin, WANG Li-fang, JIANG Lei, ZHU Dao-ben. TiO2 micro/nano-composite structured electrodes for quasi-solid-state dye-sensitized solar cells [J]. Nanotechnol, 2006, 17(9): 2090-2097.
[19] SING K S W, EVERETT D H, HAUL R A W, MOSCOU L, PIEROTTI R A, ROUQUEROL J, SIEMIENIEWSKA T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity [J]. Pure Appl Chem, 1985, 57(4): 603-619.
[20] AO Yan-hui, XU Jing-jing, FU De-gang. Deposition of anatase titania onto carbon encapsulated magnetite nanoparticles [J]. Nanotechnol, 2008, 19: 405604.
[21] YU Jia-guo, ZHOU Ming-hua, CHENG Bei, YU Huo-gen, ZHAO Xiu-jian. Ultrasonic preparation of mesoporous titanium dioxide nanocrystalline photocatalysts and evaluation of photocatalytic activity [J]. J Mole Catal A, 2005, 227(1-2): 75-80.
[22] XIN J H, ZHANG S M, QI G D, ZHENG X C, HUANG W P, WU S H. Preparation and characterization of the Bi-doped TiO2 photocatalysts [J]. React Kinet Catal Lett, 2005, 86(2): 291-298.
[23] JING Li-qiang, XIN Bai-fu, YUAN Fu-long, XUE Lian-peng, WANG Bai-qi, FU Hong-gang. Effects of surface oxygen vacancies on photophysical and photochemical processes of Zn-doped TiO2 nanoparticles and their relationships [J]. J Phys Chem B, 2006, 110(36): 17860-17865.
[24] MROWETZ M, SELLI E. H2O2 evolution during the photocatalytic degradation of organic molecules on fluorinated TiO2 [J]. New J Chem, 2006, 30(1): 108-114.
[25] YAO W F, WANG H, XU X H, CHENG X F, HUANG J, SHANG S X, YANG X N, WAN G M. Photocatalytic property of bismuth titanate Bi12TiO20 crystals [J]. Appl Catal A, 2003, 243(1): 185-190.
[26] MATOS J, LAINE J, HERRMANN J M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon [J]. Appl Catal B, 1998, 18(3-4): 281-291.
氧化铋/二氧化钛复合颗粒的制备及其光催化降解4-氯苯酚的性能
徐晶晶1, 2, 陈敏东1, 付德刚3
1. 南京信息工程大学 环境科学与工程学院 江苏省大气环境监测与污染控制高技术研究重点实验室,南京 210044;
2. 河海大学 水文水资源与水利工程科学国家重点实验室,南京 210098;
3. 东南大学 生物电子学国家重点实验室,南京 210096
摘 要:在低温条件下制备在太阳光照射下具有高光催化活性的氧化铋/二氧化钛复合颗粒。并利用XRD、SEM、BET、XPS和DRS对其进行表征。结果表明:将二氧化钛纳米颗粒沉积在氧化铋表面可形成微-纳结构,使该复合材料表现出多孔性,并提高表面羟基的含量。因此,在太阳光的激发下,氧化铋/二氧化钛复合颗粒对4-氯苯酚的催化降解能力高于纯二氧化钛和P25。
关键词: 氧化铋; 二氧化钛; 光催化; 4-氯苯酚
(Edited by FANG Jing-hua)
Foundation item: Project supported by the Scientific Research Foundation of Nanjing University of Information Science and Technology, China; Project (2010490511) supported by the Open Foundation of State Key Laboratory of Hydrology-Water Resources and Hydraulic Engineering, China
Corresponding author: XU Jing-jing, Tel: 86-25-58731090; E-mail: xujj@seu.edu.cn; xujj@nuist.edu.cn
DOI: 10.1016/S1003-6326(11)60719-X


