
Oxidation resistance of co-deposited Ni-SiC nanocomposite coating
ZHOU Yue-bo(周月波), DING Yuan-zhu(丁元柱)
Department of Materials Science and Engineering, Heilongjiang Institute of Science and Technology,Harbin 150027, China
Received 20 December 2006; accepted 20 March 2007
________________________________________________________________________________
Abstract:
Ni-6.0%SiC (mass fraction) nanocomposite coating was prepared from a nickel sulfate bath by co-electrodeposition of Ni and SiC nanoparticles in an average size of 30 nm. The oxidation at 1 000 ℃ shows that the Ni-6.0%SiC nanocomposite coating has a superior oxidation resistance compared with the pure Ni film due to the formation of SiO2 oxide particles along grain boundaries, blocking the outward diffusion of Ni and changing the oxidation growth mechanism. The effect of SiC nanoparticles on the oxidation progress was discussed in detail.
Key words:
Ni; SiC; electrodeposition; composite coating; oxidation;
________________________________________________________________________________
1 Introduction
The composite electrodeposition technique is a low-cost and low-temperature method suitable for producing metal matrix composite coatings for different purposes such as improving the wear resistance. Ni-SiC composites have been studied extensively[1-3] and commercialized successfully for the protection of mechanical parts under friction at low temperature, due to the high wear resistance and low cost of ceramic powder. The usual sizes of SiC powder in such applications are in the range of micrometers. However, as for wear resistance composite coatings used at high- temperature such as combustion engines and casting moulds[4], a good oxidation resistance is needed. Earlier works[5] suggested that at a given particle content, the refinement of SiC particles could improve the oxidation resistance, which was also proved by the recent works about the oxidation of the as-codeposited Ni-Al/Cr nano- composite coatings[6-8]. With increasing availability of SiC nanoparticles, novel Ni-SiC nanocomposite coatings were developed with enhanced wear and corrosion resistance[9-13]. However, to author’s knowledge, there were no reports about the oxidation of the codeposited Ni-SiC nanocomposite coating at high temperature. In the present work, the oxidation performance of Ni-SiC nanocomposite coatings and pure Ni film were comparatively investigated.
2 Experimental
Pure nickel specimens with the size of 15 mm×10 mm×2 mm were cut from a pure electrolytic nickel plate and then were abraded with 800# grit SiC waterproof paper. After being ultrasonically cleaned in acetone, they were electrodeposited with a Ni-SiC nanocomposite coating from a nickel sulfate bath containing certain content of pure SiC nanoparticles with an average size of 30 nm. The current density used was 3 A/dm2, the temperature 35 ℃ and the pH value 5.5-6.0. Magnetic stirring was employed to maintain the uniform particle concentration and prevent the sedimentation. For comparison, specimen of nickel with a Ni film was also deposited using the same parameters and bath but without adding SiC nanoparticles. After the deposition, the as-deposited samples were rinsed by using distilled water and then ultrasonically cleaned for analysis.
The surface morphology and composition of the produced coatings were characterized by SEM/EDAX. The mass fraction of SiC was determined by the chemical formula of SiC. Oxidation was conducted in a muffle furnace at 1 000 ℃ for 20 h. After certain period of exposure, samples were withdrawn from the furnace for weighting. After 20 h oxidation, surface and cross- sectional morphologies of the scales were investigated by SEM/EDAX.
3 Results
3.1 Microstructure
Fig.1 shows the surface morphology of the as- deposited pure Ni film and Ni-SiC nanocomposite. A regular pyramidal structure as shown in Fig.1(a) is observed on the surface of the as-deposited nickel film. However, with the addition of SiC nanoparticles, the grain size is reduced and the morphology is changed, as shown in Fig.1(b). Clearly, fine surface protrusions, as indicated by the arrow, can be seen (Fig.1(b)). EDAX analysis shows that the protrusions have higher SiC content than the other area, suggesting that they are the fresh deposits enriched in the SiC nanoparticles, while in the other areas the co-deposited SiC nanoparticles are engulfed by Ni deposits. From the cross-sectional morphology in Fig.2, it can be found that the dark SiC nanoparticles are, in general, homogeneously dispersed in the Ni-SiC nanocomposite film, although some of them form agglomerated clusters. EDAX analysis reveals that the as-deposited Ni-SiC nanocomposite contains 6.0% (mass fraction) SiC based on the chemical formula of SiC.
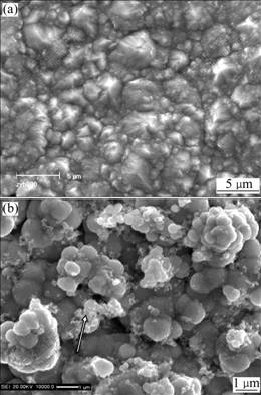
Fig.1 Surface morphologies of as-deposited pure Ni (a) and Ni-SiC nanocomposite (b)
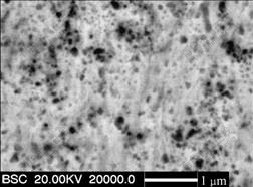
Fig.2 Cross-sectional morphology of Ni-SiC nanocomposite clearly showing homogenously dispersion of SiC nanoparticles
3.2 Oxidation performance
The kinetics of the as-deposited Ni film and Ni-SiC nanocomposite coating for 20 h oxidation at 1 000 ℃ are illustrated in Fig.3 as linear plots (Fig.3(a)) and parabolic plots (Fig.3(b)), respectively. At 1 000 ℃, the kinetics for the as-deposited Ni film and Ni-SiC nanocomposite obey the parabolic law to a good approximation for the whole duration of the test with rate constants of 3.35×10-10g2/(cm4?s) and 1.17×10-10 g2/(cm4?s), respectively. It is obvious that the Ni-SiC nanocomposite exhibits better oxidation resistance than as-deposited Ni film.
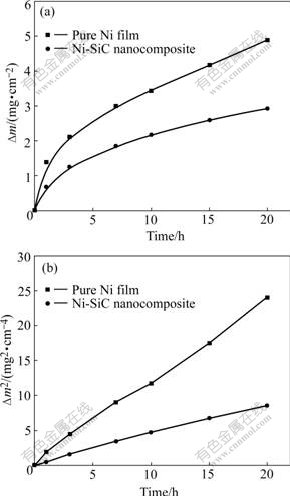
Fig.3 Dependence of mass change vs. time for various samples oxidized at 1 000 ℃ for 20 h: (a) Linear plot; (b) Parabolic plot
3.3 Surface and cross-sectional morphological investigation
XRD analysis shows that the scale formed on the two coatings is NiO. Fig.4 shows the surface morphologies of two coatings after oxidation. Faceted NiO grains with the mean size of around 10 mm appear on the as-deposited Ni film, as seen in Fig.4(a). Examination of fracture cross-sections indicates the scale consists of a relatively coarse-grained, columnar outer layer and a more porous fine-grained inner layer with a similar thickness. However, the NiO crystals formed on the Ni-SiC nanocomposite are finer, which are about 2 mm in average size (Fig.4(b)). From the corresponding cross-sectional morphology, the scales formed on Ni film exhibit a porous structure with the thickness of about 35 mm, as seen in Fig.5(a). The scales formed on Ni-SiC nanocomposite coating also exhibit a similar double-layer structure with the total thickness of about 25 mm (Fig.5(b)). However, the thickness ratio of inner layer to outer layer is about 3. EDAX results show that the outer layer is pure NiO, however gray oxides particularly along the grain boundaries exist in the inner layer, as seen in Fig.5(b). The intergranular penetration of gray oxides into the deposits also occurs. EDAX results show that the gray oxides are Si-enriched.
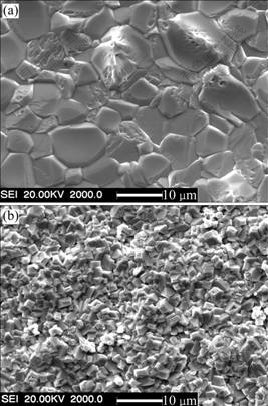
Fig.4 Surface morphologies of scales formed on Ni film (a) and Ni-SiC nanocomposite (b) oxidized at 1 000 ℃ for 20 h
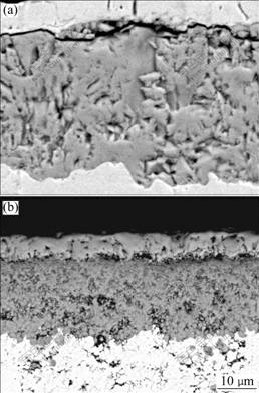
Fig.5 Cross-sectional morphologies of scales formed on Ni film (a) and Ni-SiC nanocomposite (b) oxidized at 1 000 ℃ for 20 h
4 Discussion
From Figs.1 and 2, it can be seen that the average nickel grain size of the codeposited Ni-SiC nanocomposite is smaller than that of electroplated nickel film. It seems certainly that the growth rate of NiO on the Ni-SiC nanocomposite should be at least as fast as that on nickel film[14]. However, the fact is that the growth rate of NiO scale is noticeably retarded and a fine-grain NiO scale is formed. Since the growth of Ni scale is mainly controlled by the outward diffusion of nickel cations along the oxide grain boundaries[15-16], the fine-grained NiO scale formed on Ni-SiC nano- composite coating should grow even faster than pure Ni film due to an increase in the number of grain boundaries per unit volume in the scale. However, the fact that a significant scalling rate reduction occurs, suggesting that grain-boundary diffusion of Ni cations is hindered to a great extent. Although the scales formed on the two coatings exhibit a similar duplex structure, the scales formed on the Ni-SiC nanocomposite exhibit a thicker inner layer. Investigations[15-16] showed that the outer layers formed from the outward diffusion of nickel, and the inner layers formed from the inward diffusion of molecular species of oxygen. Thus, the growth of Ni scale on Ni-SiC nanocomposite coating is mainly controlled by the inward diffusion of oxygen. From above analysis, it can be found that the addition of SiC nanoparticles in the Ni film blocks the outward diffusion of nickel cations and changes the oxidation growth mechanism, which causes a reduction of scalling rate.
At the onset of oxidation, NiO and SiO2 simultaneously nucleate, on the nanocrystalline Ni and on the SiC nanoparticles. Due to a low SiC particle content, a continuous protective SiO2 scales can not be formed by the healing of the SiO2 nuclei through their lateral growth during transient oxidation[6-8]. NiO scale grows rapidly and engulfs the SiO2 nuclei or SiC nanoparticles on the surface, so a continuous external coarse grain NiO scales without SiO2 oxides are developed. Due to the diffusion rate of Ni2+ through SiO2 is considerably less than that through NiO[5, 17], the presence of SiO2 at the metal/scale interface can have a blocking effect on the outward diffusion of Ni2+ through the NiO scale. In this way, the inward diffusion of oxygen through the outer layer controls the oxidation progress of Ni-SiC nanocomposite coatings. At the same time, the SiC particles in the electrodeposit react with inward diffused oxygen, mainly along the grain boundaries, to form SiO2 oxide particles in the inner layer of scale or in the deposits. The SiO2 particles at the grain boundary can play roles in inhibiting the grain growth by the ‘solute-drag’ effect or pinning[14, 17], which causes fine-grained oxides.
5 Conclusions
1) Ni-6.0%SiC (mass fraction) nanocomposite exhibits improved oxidation resistance compared with pure Ni film.
2) With the addition of SiC nanoparticles, the inward diffusion of oxygen controls the oxidation progress of Ni-SiC nanocomposite coating.
3) It may be possible to produce a Ni-SiC nanocomposite coating with a high SiC particle content, which could form a continuous protective SiO2 scale through the control of the electrodeposition parameters.
References
[1] ZIMMERMAN A F, PALUMBO G, AUST K T, ERB U. Mechanical properties of nickel silicon carbide nanocomposites [J]. Mater Sci Eng A, 2002, A328: 137-146.
[2] HOU K H, GER M D, WANG L M, KE S T. The wear behaviour of electro-codeposited Ni-SiC composites [J]. Wear, 2002, 253: 994-1003.
[3] AAL A A, IBRAHIM K M, HAMID Z A. Enhancement of wear resistance of ductile cast iron by Ni-SiC composite coating [J]. Wear, 2006, 260: 1070-1075.
[4] ORLOVSKAJA L, PERJENE N, KURTINAITIENE M, SURVILIENE S. Ni-SiC composite plated under a modulated current [J]. Sur Coat Technol, 1999, 111: 234-239.
[5] STOTT F H, ASHBY D J. The oxidation characteristics of electrodeposited nickel composites coating containing silicon carbide particles at high temperature [J]. Corros Sci, 1978, 18: 183-198.
[6] ZHANG Y, PENG X, WANG F. Development and oxidation at 800 ℃ of a novel electrodeposited Ni-Cr nanocomposite film [J]. Materials Letters, 2004, 58: 1134-1138.
[7] ZHOU Y, PENG X, WANG F. Oxidation of a novel electrodeposited Ni-Al nanocomposite film at 1 050 ℃ [J]. Scripta Mater, 2004, 50: 1429-1433.
[8] ZHOU Y, PENG X, WANG F. Size effect of Al particles on the oxidation of electrodeposited Ni-Al composite coatings [J]. Oxid Met, 2005, 64: 169-183.
[9] ZIMMERMAN A F, CLARK D G, AUST K T, ERB U. Pulse electrodeposition of Ni-SiC nanocomposite [J]. Materials Letters, 2002, 52: 85-90.
[10] GYFTOU P, STROUMBOULI M, PAVLATOU E A, ASIMIDIS P, SPYRELLIS N. Tribological study of Ni matrix composite coatings containing nano and micro SiC particles [J]. Electrochimica Acta, 2005, 50: 4544-4550.
[11] LEKKA M, KOULOUMBI N, GAJO M, BONORA P L. Corrosion and wear resistant electrodeposited composite coatings [J]. Electrochimica Acta, 2005, 50: 4551-4556.
[12] GARCIA I, FRANSAER J, CELIS J P. Electrodeposition and sliding wear resistance of nickel composite coating containing micro and submicron SiC particles [J]. Sur Coat Technol, 2001, 148: 171-178.
[13] LEE H K, LEE H Y, JEON J M. Codeposition of micro- and nano-sized SiC particles in the nickel matrix composite coatings obtained by electroplating [J]. Sur Coat Technol, 2007, 201: 4711-4717.
[14] PENG X, PING D, LI T, WU W. Oxidation behavior of a Ni-La2O3 codeposited film on nickel [J]. J Electrochem Soc, 1998, 145: 389-398.
[15] ATKINSON A, TAYLOR R I, GOODE P D. Transport processes in the oxidation of Ni studied using tracers in growing NiO scales [J]. Oxid Met, 1979, 13: 519-543.
[16] ATKONSON H V. Evolution of grain structure in nickel oxide scales [J]. Oxid Met, 1987, 28: 353-389.
[17] REIDAR H, ANETTE E G, CHRISTIAN R S. Effects of sol-gel-derived silica coatings on high-temperature oxidation of Ni [J]. Oxid Met, 2001, 56: 453-465.
______________________________
Foundation item: Project(06-13) supported by the Scientific Research Startup Foundation of Heilongjiang Institute of Science and Technology, China
Corresponding author: ZHOU Yue-bo; Tel: +86-451-88036526: E-mail: zhouyuebo760309@163.com; ybzhou@imr.ac.cn


