
High-temperature oxidation behavior of Nb67-xW15Si18Hfx(x=0, 5 and 10) alloys
JIANG Rong-li(姜荣丽)1, LIU Dong-ming(刘东明)2, SHA Jiang-bo(沙江波)1, MA Yue(马 岳)1
1. School of Materials Science and Engineering,
Beijing University of Aeronautics and Astronautics, Beijing 100083, China
2. School of Materials Science and Engineering, South Campus, Shandong University, Ji'nan 250061, China
Received 28 July 2006; accepted 15 September 2006
Abstract:
The oxidation behaviors of Nb67-xW15Si18Hfx (x=0, 5, 10) alloys were studied at 1 250 ℃ in air. It is found that the Nb67W15Si18 alloy has the best oxidation resistance among the three alloys; and Hf addition is harmful to the oxidation resistance of the Nb67W15Si18 alloy. The oxides formed on the Nb67W15Si18 alloy are mainly Nb12WO33 and NbO2, and that on the Nb62W15Si18Hf5 and Nb57W15Si18Hf10 alloys is Nb2O5. Effect of Hf on the oxidation behavior of the Nb67-xW15Si18Hfx alloys has been discussed based on microstructures and kinetics of oxidation.
Key words:
Nb-Si based alloys; Ni67-xW15Si18Hfx alloys; high-temperature oxidation; oxidation resistance; oxide;
1 Introduction
Niobium-silicide based composites are potential candidates for aircraft engine components because of their high melting temperature, low density and relatively high strength at elevated temperatures[1-5]. Significant work on mechanical properties has been focused on optimizing the room-temperature fracture toughness and high-temperature strength[6-10]. In the previous work[11], a typical Nb-Si alloy, Nb67W15Si18, showed excellent high-temperature strength; and its 0.2% yield compressive strength was as high as 880 MPa at 1 200 ℃ and 570 MPa at 1 500 ℃. Its room temperature fracture toughness can be improved by Hf addition, from 5.1 MPa·m1/2 to 7.2 MPa·m1/2, when Hf content changes from 0% to 15%(mole fraction). However, the oxidation behaviors of the Nb67-xW15Si18Hfx (x=0, 5, 10) alloys are unknown. In the present paper, the Nb67-xW15Si18Hfx (x=0, 5, 10) alloys were oxidized at 1 250 ℃ in air to investigate the oxidation behavior and mechanism and probe the balance tendency among strength, toughness and oxidation behavior of Nb-Si based alloys.
2 Experimental
Three alloys, Nb67-xW15Si18Hfx (x=0, 5, 10), were
prepared by arc melting in a vacuum from high purity metals Nb(99.6 %), W(99.9 %), Si(99.999 %) and Hf (99.9 %). The ingots were arc-melted five or six times to homogenize the compositions. Then the ingots were annealed at 1 750 ℃ for 50 h in a vacuum of 1×10-4 Pa followed by furnace cooling to room temperature. The specimens for oxidation were cut into sizes of 6 mm×5 mm×3 mm from the ingots, and ground to an 800 grit polisher and washed in acetone.
Isothermal static oxidation experiment was conducted at 1 250 ℃ for 1 h, 3 h, 5 h and 7 h in air. Oxidation rates were obtained by measuring the mass gains of the samples. The mass gains were weighed by an electronic balance with an accuracy of 0.1 mg. A scanning electron microscope (HITACHI S-3500N) with energy-dispersive spectrometer (Oxford INCA) was used to analyze the surface morphology and cross section of the specimens after oxidation test. The compositions of the oxides were analyzed by X-ray diffraction(XRD) technique.
3 Results and discussion
XRD analysis shows that the phase structures of the three alloys contain Nb solid solution (hereafter referred to as NbSS) and (Nb, W, Hf)5Si3 silicide (hereafter referred to as M5Si3). Fig.1 shows the typical micro- structure of the three alloys, in which the bright and dark areas correspond to the NbSS and the M5Si3 phases, respectively. With 18%(mole fraction) Si, the Nb67W15- Si18 alloy approaches the eutectic composition, and the microstructure should be composed of eutectoid composition according to the binary Nb-Si phase diagram. However, the Nb67W15Si18 alloy annealed at 1 750 ℃ for 50 h shows more primary NbSS than the eutectic Nb-18Si alloy. This result suggests that the W addition may give rise to a shift of the eutectic line towards the Si-rich corner of the phase diagram. EDS results reveal that most of W is dissolved in the NbSS, while most of Hf is dissolved in the M5Si3. The annealed Nb67W15Si18 alloy shows a microstructure composed of the primary NbSS and the eutectic containing the secondary NbSS and the M5Si3 silicide. Compositions for the phases in the Nb57W15Si18Hf10 alloy annealed at 1 750 ℃ for 50 h are shown in Table 1.
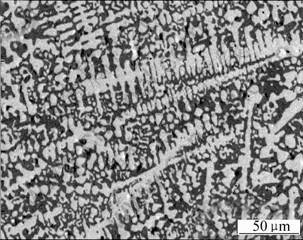
Fig.1 Microstructure (BSE image) of Nb67W15Si18 alloy annealed at 1 750 ℃ for 50 h
Table 1 Compositions for phases of Nb57W15Si18Hf10 alloy annealed at 1 750 ℃ for 50 h(mole fraction, %)
The oxidation kinetics curves of the three alloys at 1 250 ℃ for 7 h are shown in Fig.2. The mass gains of the 5Hf and 10Hf samples are larger than those of the Hf-free sample under the same oxidation conditions. The 10Hf sample shows the highest mass gain of 111.87 mg/cm2 after oxidation for 7 h. The oxidation rates of the 0Hf, 5Hf and 10Hf alloys are 5.97 mg/(cm2·h), 11.30 mg/(cm2·h) and 15.98 mg/(cm2·h), respectively. It can be concluded that with increasing Hf content the oxidation rate increases. After oxidation at 1 250 ℃ for 7 h, the oxide layer is light yellow and some oxides scale off the alloys.
Fig.3 shows the surface morphologies of the three oxidized alloys. The microstructures and compositions of the oxide scales were analyzed by SEM/EDS and XRD.
The oxides of the Nb67W15Si18 alloy are cluster rods (average diameter about 1-4 μm), which grow outwardly from the inner parts of the oxide scale. At 5%(mole fraction) Hf content, the oxide surface is covered partially by Hf-rich particles with a size of 1-2 μm. For the 10%(mole fraction) Hf alloy, nearly 90% of the surface is covered by particle phase, as shown in Fig.3(c). The XRD results in Fig.4 indicate that the oxides in the Nb67W15Si18 alloy mainly consist of Nb12WO33 and NbO2, while those of the 5Hf and 10Hf alloys are Nb2O5 and WO3. It can also be concluded from Fig.3 that the morphology of the 5Hf alloy surface is similar to that of the 0Hf alloy surface except that some Hf-rich particles appear on the oxide rods in the 5Hf alloy. The oxides, Nb12WO33 and NbO2, in the Nb67W15Si18 alloy, are distributed in the scale. For the 10Hf alloy, the oxide surface is entirely covered by particles with some cracks, which offers channels for the diffusion of oxygen, and then the oxide Nb2O5 grows outwardly through the cracks.
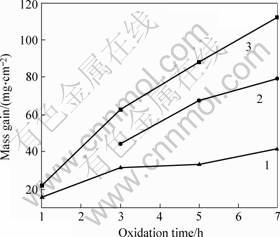
Fig.2 Kinetics curves of isothermal static oxidation of Nb67-xW15Si18Hfx alloys at 1 250 ℃: 1 Nb67W15Si18; 2 Nb62- W15Si18Hf5; 3 Nb57W15Si18Hf10
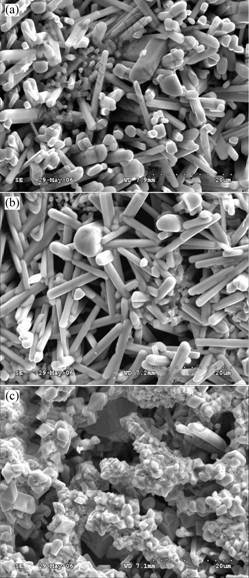
Fig.3 Surface morphologies (SEM/SE) of Nb67-xW15Si18Hfx (x=0, 5, 10) alloys oxidized at 1 250 ℃ for 7 h in air:
(a) Nb67W15Si18; (b) Nb62W15Si18Hf5; (c) Nb57W15Si18Hf10
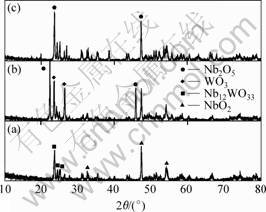
Fig.4 X-ray diffraction patterns of Nb67-xW15Si18Hfx alloys oxidized at 1 250 ℃ for 7 h: (a) Nb67W15Si18; (b) Nb62W15Si18Hf5; (c) Nb57W15Si18Hf10
The cross sectional morphologies of the oxide scales of the Nb67-xW15Si18Hfx alloys are illustrated in Fig.5. There are three distinct layers on the cross section, i.e., oxide layer, transition layer and substrate. The crack along the oxide/transition interface indicates that the oxide and the substrate are not well bonded, and the crack becomes wider with the increase of Hf, so the oxides scale off the substrate after oxidation. The transition on the 0Hf alloy is about 20 μm, and it is larger than 20 μm with the addition of Hf element. Table 2 and Table 3 show the mass fraction of oxygen in the NbSS and M5Si3 phases. It can be seen that oxygen dissolved in the M5Si3 is more than that in the NbSS, therefore, the dark areas in the transition layer become thicker than in the substrate. In view of the oxidation thermodynamics, the Gibbs free energy for the oxides Nb2O5 and NbO2 formation is much lower than that for the oxides HfO2 and SiO2. Accordingly, when oxidation occurs, the NbSS phase is oxidized preferentially and forms Nb2O5 and NbO2 on the surface because of its high content of Nb. In the transition layer, the silicide phase is more than the NbSS phase.
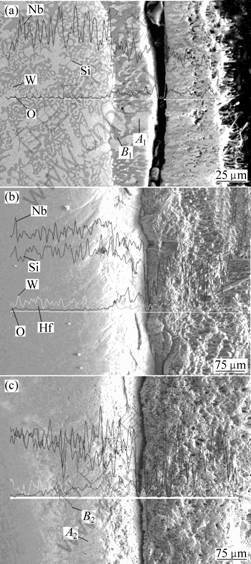
Fig.5 SEM/SE images of cross-sectional morphologies of Nb67-xW15Si18Hfx alloys oxidized at 1 250 ℃ for 7 h in air:
(a) Nb67W15Si18; (b) Nb62W15Si18Hf5; (c) Nb57W15Si18Hf10
Table 2 Mass fractions of elements in transition layer of Nb67W15Si18 alloy(A1 and B1 respectively correspond to the NbSS and M5Si3 areas in Fig.5 (a).)

Table 3 Mass fractions of elements in transition layer of Nb57W15Si18Hf10 alloy(A2 and B2 respectively correspond to the NbSS and M5Si3 areas in Fig.5 (c).)

The fine Pt markers placed on the top of the samples prior to oxidation are seen in the upper parts of the scale after oxidation, as shown in Fig.6. This implies that oxidation proceeds via the inward diffusion of oxygen. The oxide Nb2O5 grows continually from the substrate of the oxide layer. For the 10Hf alloy, most of Hf is separated out to the oxide surface and broken up as particles on the upper parts of the scale, as shown in Fig.3(c) and Fig.5, while Nb is oxidized at lower parts of the scale and grows outwardly through the Hf-rich particles.
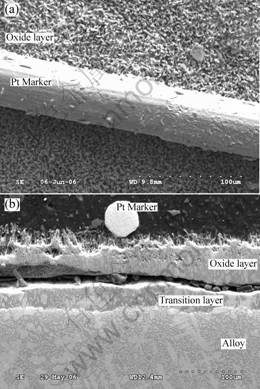
Fig.6 SEM/SE images of Nb67W15Si18alloy with Pt markers on oxide layer after oxidation at 1 250 ℃ for 3 h in air:
(a) Surface image; (b) Cross-section image
4 Conclusions
1) Hf is harmful to the oxidation resistance of the Nb67-xW15Si18Hfx alloys. The oxidation rate is accelerated by increasing Hf content.
2) In oxidation procedure, oxygen diffuses inwardly form the oxide Nb2O5, and Hf diffuses outwardly and conglomerates on the oxide layer in the form of particle. The oxidation mechanism of the Nb67-xW15Si18Hfx alloys is internal oxidation.
References
[1] BEWLAY B P, JACKSON M R, ZHAO J C, SUBRAMANIAN P R. A review of very high-temperature Nb-silcide-based composites[J]. Metall Mater Trans A, 2003, 34A(10): 2043-2052.
[2] BEWLAY B P, JACKSON M R, ZHAO J C, SUBRAMANIAN P R, MENDIRATTA M G, LEWANDOWSKI J J. Ultrahigh-temperature Nb-silicide-based composites[J]. MRS Bulletin, 2003, 28(9): 646-653.
[3] BEWLAY B P, LEWANDOWSKI J J, JACKSON M R. Refractory metal-intermetallic in-situ composites for aircraft engines[J]. JOM, 1997, 49(8): 44-45
[4] DING Xu, GUO Xi-ping. The research development of Nb-silcide-based composites[J]. Materials Review, 2003, 17(11): 60-62.
[5] QU Shi-yu, WANG Rong-ming, HAN Ya-fang. The research development of Nb-Si based intermetallic alloys[J]. Materials Review, 2002, 16(4): 31-34.
[6] SHA Jiang-bo, HIRAI H, TABARU T, KITAHARA A, UENO H, HANADA S. Mechanical properties of as-cast and directionally solidified Nb-Mo-W-Ti-Si in-situ composites at high temperatures[J]. Metallurgical and Materials Transactions A, 2003, 34(1): 85-94.
[7] SHA Jiang-bo, HIRAI H, TABARU T, KITAHARA A, UENO H, HANADA S. High-temperature strength and room-temperature toughness of Nb-W-Si-B alloys prepared by arc-melting[J]. Materials Science and Engineering A, 2004, 364(1-2): 151-158.
[8] SHA Jiang-bo, HIRAI H, TABARU T, KITAHARA A, UENO H, HANADA S. Effect of carbon on microstructure and high-temperature strength of Nb-Mo-Ti-Si in situ composites prepared by arc-melting and directional solidification[J]. Materials Science and Engineering A, 2003, 343(1-2): 282-289.
[9] KIM J, TABARU T, SAKAMOTO M, HANADA S. Mechanical properties and fracture behavior of an NbSS/Nb5Si3 in-situ composite modified by Mo and Hf alloying[J]. Materials Science and Engineering A, 2004, 372(1-2): 137-144.
[10] MURAYAMA Y, HANADA S. High temperature strength, fracture toughness and oxidation resistance of Nb-Si-Al-Ti multiphase alloys[J]. Science and Technology of Advanced Materials, 2002, 3(2): 145-156.
[11] LIU Dong-ming. Microstructure and mechanical behaviors of ultra-high temperature Nb-Si-W-X alloy[D]. Beijing: Beijing University of Aeronautics and Astronautic, 2006.
(Edited by PENG Chao-qun)
Foundation item: Project(50671002) supported by the National Natural Science Foundation of China
Corresponding author: JIANG Rong-li; Tel: +86-10-82315989(300); E-mail:apple_j@126.com


