J. Cent. South Univ. (2013) 20: 480–487
DOI: 10.1007/s11771-013-1509-8
Optimization of Cr(VI) bioremediation in contaminated soil using indigenous bacteria
LI Qian(李倩)1, 2, YANG Zhi-hui(杨志辉)1, CHAI Li-yuan(柴立元)1, WANG Bing(王兵)2,
XIONG Shan(熊珊)1, LIAO Ying-ping(廖映平)1, ZHANG Shu-juan(张淑娟)1
1. Institute of Environmental Science & Engineering, School of Metallurgical Science and Engineering,
Central South University, Changsha 410083, China;
2. Hunan Research Institute of Nonferrous Metals, Changsha 410015, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Central South University Press and Springer-Verlag Berlin Heidelberg 2013
Abstract:
Bench-scale soil column experiments were carried out to evaluate the effectiveness of Cr(VI) bioremediation process in soils by using indigenous bacteria with the addition of bacteria nutrient media. Effects of particle size, spray intensity, initial Cr(VI) concentration, circulation mode and soil depth on Cr(VI) remediation were studied. Results show that soils after 6 d remediation with spray intensity controlled in the range of 29.6–59.2 mL/min could well fulfill the requirement of concrete aggregate and roadbed material usage, for the leaching toxicity concentration of the Cr(VI) in treated soils under the chosen condition is far less than 5 mg/L. The leaching toxicity and fractions of both hexavalent chromium and trivalent chromium from remediated soils were determined and compared with that of untreated soil. The results show that water soluble Cr(VI) declines from 1520.54 mg/kg to 0.68 mg/kg, exchangeable Cr(VI) decreases from 34.83 mg/kg to 0.01 mg/kg and carbonates-bonded Cr(VI) falls from 13.55 mg/kg to 0.68 mg/kg. Meanwhile, a corresponding increase in carbonate-bonded Cr(III), Fe and Mn oxides-bonded Cr(III) and organic matter-bonded Cr(III) are found. It reveals that indigenous bacteria can leach out water soluble Cr(VI), exchangeable Cr(VI) and carbonates-bonded Cr(VI) from contaminated soil followed by converting into carbonate-bonded Cr(III), Fe and Mn oxides-bonded Cr(III), organic matter-bonded Cr(III) and residual Cr(III).
Key words:
bioremediation; Cr(VI) pollution control; indigenous bacteria;
1 Introduction
Chromium contaminated sites spread widely since the anthropogenic inputs of chromium from industrial processes such as tannery, resistant alloys, mining etc, have increased rapidly [1]. Proper treatment of these areas is of great concern recently. Hexavalent chromium [Cr(VI)] and trivalent chromium [Cr(III)] are the major polluting states among the nine valence states ranging from –2 to +6 in soil environment [2]. Cr is essential in certain amounts, but it becomes toxic at higher doses. Hexavalent chromium is highly soluble, highly toxic, carcinogenic, and mutagenic whereas trivalent chromium is relatively less toxic and less mobile [3–4]. Cr(VI) has been listed as a priority pollutant and a human carcinogen by the US Environmental Protection Agency.
The conventional physico-chemical remediation methodologies for soils contaminated with Cr(VI) require high energy and plenty of chemical re-agent when applied to large scale [4] , what’s more, these methods will be inefficient when the Cr(VI) is in low concentration and it could not remove chromium completely [5]. Therefore, these methods do not economically feasible. Consequently, the economical and environmental friendly remediation of Cr(VI) contaminated sites is in urgent need. Bioremediation is one of the promising methods to clean up the contaminated sites. The strategy of bioremediation is to detoxify Cr(VI) in the soil to less soluble trivalent form via the normal function of the microbial metabolism [6]. Microorganisms including bacteria, fungi, algae and yeast uptake metal either through bioaccumulation or through biosorption [7]. Recently, attention has been paid on the bioremediation of contaminated soil using bacteria. Many Cr(VI) reduction microbes have been isolated and characterized from either aerobic or anaerobic conditions [8–13]. Cheng and Li [9] isolated eight isolates from soil samples of iron mineral area, one of which, the MDS05 showed great promise for use in Cr(VI) detoxification under a wide range of environmental conditions. Lee et al [11] enriched the bacteria in the chromium contaminated sediment, and found that this bacteria reduced 34% of dissolved Cr(VI) in the sediment. Martins et al [13] discovered that the uranium (VI) removing communities also have the ability to remove chromium (VI), and be the first to propose the members of Enterobacteriaceae and Rhodocyclaceae families that could remove chromium and uranium.
Though many studies reported on bioremediation of Cr(VI) contaminated soil, most of them require the addition of exogenous microbes or the microbes isolated from the contaminated soil but enriched and cultivated before applying in the actual bioprocess [14–16], which would bring about the ecological unbalance. More and more researchers on soil remediation started to focus on indigenous microorganisms. Colin et al [3] summarized the indigenous microbial that has effect on the chromium contaminated medium, and tried to explain the mechanisms. Jastin et al [17] reported that they isolated three kinds of indigenous bacteria from the water of chromium mining sites at Sukinda Valley, which showed a considerable enhancement in Cr(VI) bioreduction rate, and successfully provided a choice for chromium mine sites remediation. Chai et al [18] reported in 2009 that they isolated and identified an indigenous bacteria from soils contaminated by chromium-containing slag and found that once culture medium was added into the contaminated soil, the concentration of the water soluble Cr(VI), exchangeable Cr(VI) and carbonate-bonded Cr(VI) decreased. Most of the researches were focused on the discovering, isolation and identification of the specific indigenous bacteria, few has the actual application to the contaminated sites. The indigenous bacteria discovered by Chai et al [18] have been applied in the remediation of Cr(VI) contaminated sites in west of Hunan province, China. And it is the first time to report that we conducted a bench-scale study and confirmed the technological parameters of the indigenous bacteria discovered by Chai et al [18] before starting the pilot scale and the field scale remediation.
In the present work, bench-scale column experiments were conducted, in continuation of the early study [18], to evaluate the effectiveness of Cr(VI) bioreduction process by indigenous bacteria and to provide the back of technology for the field applying. The influencing factors including soil particle size, spray intensity of the washing liquid, initial Cr(VI) concentration in soil and circulation mode were studied. In addition, the bioremediation of all Cr(VI) fractions including water soluble Cr(VI), exchangeable Cr(VI), carbonate-bonded Cr(VI), Fe and Mn oxides-bonded Cr(VI), organic matter-bonded Cr(VI) and residue Cr(VI) were also studied. In order to make clear the migration of Cr(VI), all the mentioned fractions above of Cr(III) were analyzed. Leaching toxicity concentrations of original soil and the treated soils were tested to make sure whether the remediation was successful and provide feasible data support for the reuse of treated soils.
2 Materials and methods
2.1 Soil sample
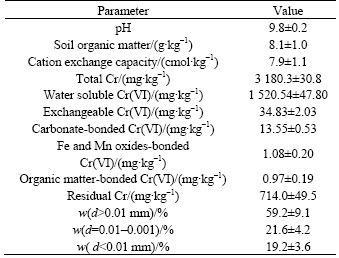
Contaminated soil for the present work was collected from a steel-alloy factory in Hunan province (27°75′N; 112°50′E), China. Soil samples were taken from the surface layer (0–20 cm) of the sites under the chromium-containing slag pile. After air drying, roots and rocks in the soil were removed. The samples were mixed well before they were used in the remediation experiments. The physico-chemical characteristics of the contaminated soil are presented in Table 1.
Table 1 physical and chemical properties of tested soils
2.2 Nutrient media
The nutrient medium for microorganism culture consisted of 5 g tryptone, 5 g yeast extract in 1 L distill water. The pH was maintained at 9.8 by using HCl or NaOH. All media were autoclaved at 121°C for 30 min prior to use.
2.3 Experimental methods
In order to study the influencing factors in the process of bioremediation of Cr(VI) contaminated soils, experiments were carried out in columns. A schematic diagram of the column recycle leaching is shown in fig. 1. The whole structure consists of a PVC reservoir, columns of organic glass, a pump, leachate collectors and PVC tubes. The column is 12 cm in diameter and 80 cm in height with four sample collection ports located at 20, 40, 60, 80 cm from the top. The water in the reservoir was heated to a certain temperature and pumped running over the sideward space of the inner column to ensure a constant temperature for the bioremediation experiments. Filter media consisting of boulders with an average size of 2 cm were packed to a height of 5 cm from the bottom over which the contaminated soil was loosely packed. The sterilized nutrient medium in the leachate collector was pumped to the top of the column with certain speed, and poured through a spray device into the contaminated soil. The leachate finally flowed into the collector to ensure the circulation. Soil samples were collected from various sampling ports and analyzed for Cr(VI) every day. The chromium fractions were determined before and after the bioremediation experiments. An equal amount of distilled water was added to the system at each time to complement the vaporization loss. The whole experiment was performed under a condition of (25±3)°C and initial pH of 10.0.
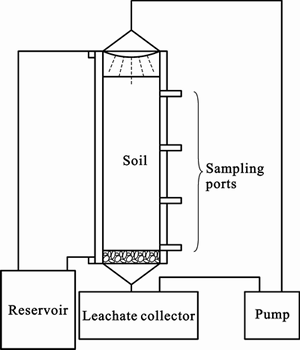
Fig. 1 schematic diagram of colum recycle leaching
2.4 Analytical procedures
For the extraction of the total chromium from soil, hydrochloric acid-nitric acid-hydrofluoric acid-perchloric acid digestion method was employed. The soluble hexavalent chromium was extracted with distilled water at 1:10 ratio of soil (passed through 150 mm sieve) to water by shaking for 1 h. The hexavalent chromium concentration was measured colorimetrically at 540 nm by reacting with diphenyl carbazide in acidic conditions.
The modified Tessier sequential extraction procedure [18–19] and inductively coupled plasma atom emission spectrum (ICP-AES) was adopted to determine the chromium fractions.
3 Results and discussion
3.1 Effect of particle size
In order to determine the effect of particle size of soils on Cr(VI) reduction, studies were carried out using autoclaved nutrient medium and soils with different particle sizes and the results are presented in Fig. 2. As expected, the particle size of soil had a significant impact on the processes for Cr(VI) leaching and reduction. It was confirmed that the indigenous bacteria has the ability to remove Cr(VI) from soil. After 6 d remediation, water soluble Cr(VI) concentration in soils dropped from 1 520.54 mg/kg to 2.05 mg/kg in the fraction of particle size <1 cm, and removal rate of water soluble Cr(VI) reached 99.87%. Yet in the fraction of particle size >4 cm, water soluble Cr(VI) concentration left 172.60 mg/kg in the treated soil, and the removal rate of water soluble Cr(VI) was 88.65%. This may be due to the fact that minor particle size of soils contaminated by the chromium-containing slag could encourage Cr(VI) dissociation and expose Cr(VI) to the interface of solid and liquid phases thus enlarge the contact area and facilitate the reaction process [20]. What’s more, the removal rate of water soluble Cr(VI) in 1–2 cm fraction was similar to that of particle size <1 cm. Hence, soils in 0–2 cm fractions would be appropriate for the bioremediation application and were used in latter experiments.
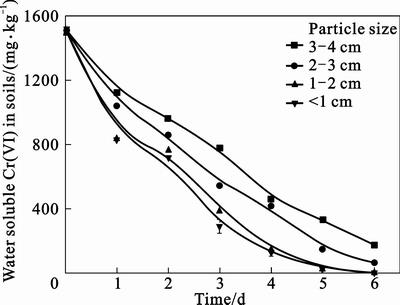
Fig. 2 Removal of water soluble Cr(VI) for different particle sizes
It was also observed that the leaching speed of Cr(VI) varied under different periods for each curve. Take the curve with particle size <1 cm as an example, in the first 2 d, since it was the adaptation period for indigenous bacteria in the test soil, at which the oxidation-reduction potential of the leaching system was at a low level, the average leaching rate was just
13.32% each day. While in the intermediate 2 d, the indigenous bacteria grew powerfully, as they were already adapted to the circumstance and entered the logarithmic phase and then stationary phase, and the oxidation-reduction potential of the leaching system rose sharply. Moreover, most Cr(VI) uncovered to the dense oxidation-reduction atmosphere, making way for Cr(VI) dissolve and to react with the indigenous bacteria. These two days were the high-speed leaching periods, its average leaching rate peaked 32.38% each day. The last 2 d was the decline phase for Cr(VI) leaching. At this period, the atmosphere was still comparatively thick and the bacteria grew fast, whereas the exposure of Cr(VI) to the surroundings decreased. Hence, the leaching speed was fairly slow, the average leaching rate was only 4.3% each day.
Soil bioremediation using indigenous micro- organisms under different particle sizes shared the same change trend of different Cr species. As shown in table 2, after the bioremediation, total Cr(VI) concentration in soils declined in different particle size fractions, while different species changed differently. The results indicated that the addition of culture medium to the contaminated soils for 6 d could remove water soluble Cr(VI) by 99.84%, exchangeable Cr(VI) by 89.71%,and carbonate-bonded Cr(VI) by 97.85%. Meanwhile, the concentration of carbonate-bonded Cr(III), Fe and Mn oxides-bonded Cr(III), organic matter-bonded Cr(III) and residual Cr(III) increased respectively during the remediation process, and there was no significant increase in concentrations of water soluble Cr(III) and exchangeable Cr(III). The results revealed that indigenous bacteria leached out water soluble Cr(VI), exchangeable Cr(VI) and carbonate-bonded Cr(VI) from contaminated soil and changed them into carbonate-bonded Cr(III), Fe and Mn oxides-bonded Cr(III), organic matter-bonded Cr(III) and residual Cr(III).
Leaching toxicity of the original soil and remediated soils with different particle sizes were also investigated. Results are presented in table 3. It was evident that soils with particle size <2 cm could well fulfill the requirement of Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity [21] and even meet the requirement of concrete aggregate and roadbed material usage (0.5 mg/L) according to environmental protection technical specifications for pollution treatment of the chromium residue after bioremediation [22].
Table 2 change of different Cr species in soils with different particle sizes
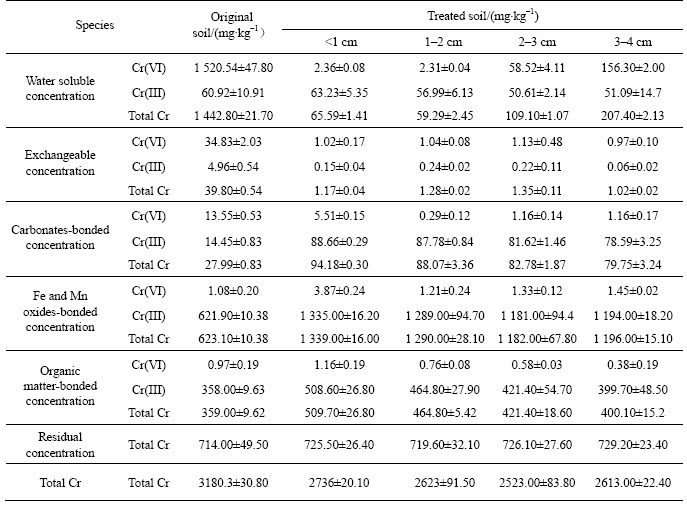
Table 3 Leaching toxicity of remediated soils with different particle sizes

3.2 Effect of spray intensity
Spray intensity has a significant impact on the biomass in the biosystem [23]. The effect of spray intensity on Cr(VI) remediation was studied at three different flow rates (7.4, 29.6 and 59.2 mL/min) under aerobic conditions. The results are shown in Fig. 3. It was observed that the reduction rate of Cr(VI) increased with the increment of the washing speed in certain extent, however, since the speed continuously increased to a certain level, the reduction rate kept constant. 9 d were needed for the bioremediation with flow rate of 7.4 mL/min, while 6 d were needed for 29.6 mL/min. Hence, the improvement of flow rate enhanced the reduction rate of Cr(VI). But when the flow rate was added to 59.2 mL/min, the time needed for effective reduction of Cr(VI) remained the same with that of for 29.6 mL/min. It was because that leaching procedure was part of the solution diffusion process [24]. As the flow rate raised, the circulation of the leachate was accelerated, and the mass transfer was enhanced. This created condition for the diffusion of Cr(VI) since the concentration gradient between the reactant and the resultant in the solid-liquid interface was shortened. Yet the increment of the spray intensity would encourage the leaching out of the impurities in the chromium contaminated soils, consequently affected the further reduction of Cr(VI). So, it can be concluded that the optimum spray intensity ranged from 29.6 to 59.2 mL/min in which the water soluble Cr(VI) effectively leached out and the reduction rate was considerable fast, and here the moderate spray intensity assured an ideal reduction rate and avoided bacteria washing out of the column.
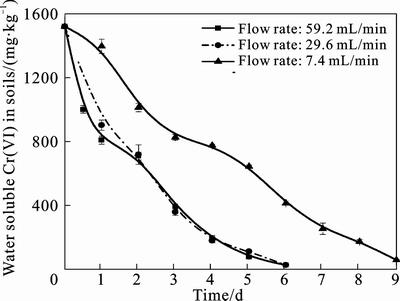
Fig. 3 Cr(VI) concentration change in soils with different spray intensities
Speciation analysis of the detoxicated soils under different flow rates as well as the original soil is listed in table 4. The trend of the results was similar with that of table 3. The difference was that the decrement of total chromium for different spray intensities was dissimilar. The slower the flow rate was, the more the decrement of total chromium will be. It may be because that Cr(VI) in soils changed into Cr(III) via bioreduction, and Cr(III) along with the washing liquid migrated to the leachate collector. The slower flow rate allowed the Cr(III) sediment to stay longer in the collector, which meant there would be less chromium in the treated soil. Therefore, slower flow rate possessed larger decrement.
Results of leaching toxicity of the remediated soils with different spray intensities are presented in table 5. It revealed that the treated soils with spray intensity controlled within 29.6–59.2 mL/min satisfied the requirement of concrete aggregate and roadbed material usage for chromium [22] . However, soils bioremediated with spray intensity of 7.4 mL/min can not reach the requirement for chromium according to Identification standards for hazardous wastes—Identification for extraction toxicity [21].
3.3 Effect of initial Cr(VI) concentration
In order to determine the influence of initial Cr(VI) concentration on microbial Cr(VI) detoxification, the change of water soluble Cr(VI) in different contaminated levels: slightly contaminated soil, heavily contaminated soil and slag mixed soil were investigated. As shown in fig. 4, time needed to remediate the contaminated soils became longer as the initial Cr(VI) concentration raised. Meanwhile, the average daily removal rate of slightly contaminated soil was faster than that of the heavily contaminated soils. This was because that Cr(VI) restrained the growth and reproduction of indigenous bacteria. And the higher Cr(VI) concentration contributed more in the restrain effect.
Leaching toxicity of the original and remediated soils with respect to different contaminated levels was studied. Results are presented in table 6. Three types of the treated soils could be used as concrete aggregate and roadbed material according to Environmental protection technical specifications for pollution treatment of the Chromium Residue [23].
Table 4 Changes of different Cr species in soils with different spray densities
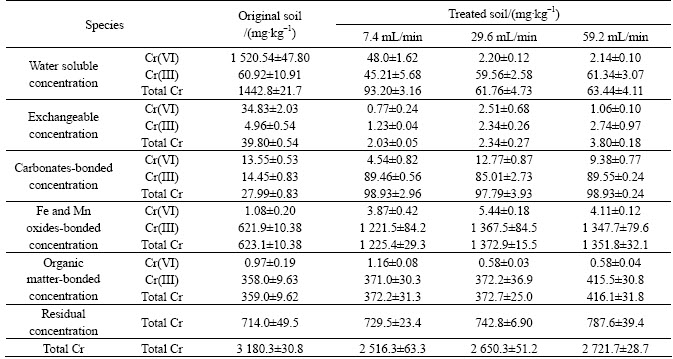
Table 5 Leaching toxicity of remediated soils with different spray densities
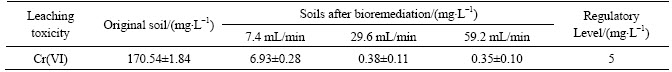
Table 6 Leaching toxicity of remediated soils with different initial Cr(VI) concentrations

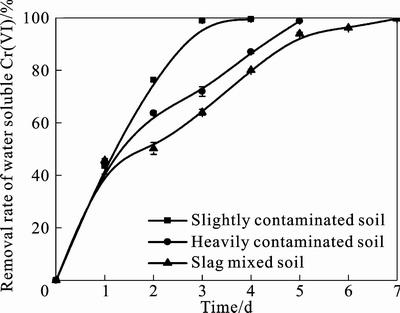
Fig. 4 Cr(VI) removal rate in soils with different initial Cr(VI) concentrations
3.4 Effect of depth
Soil samples (particle size <1 cm) at different depths were taken from the columns and analyzed for Cr(VI). The results are presented in Fig. 5. The soil samples collected from the columns revealed that there was no water soluble Cr(VI) presence at different depths after 6 d. The Cr(VI) reduction rate decreased with the increase of depth. This may be due to the following three points. 1) the washing media in the circulation possesses much indigenous microorganisms which would stay more in the surface to 20 cm depth than in the 20–80 cm in the leaching procedure; 2) Soils in the 0–20 cm could hold more oxygen in the liquid phase than the soils beneath 20 cm, therefore, bacteria in this area grew faster; 3) Part of the Cr(VI) in the 0–20 cm soils was migrated to the subsoil with the help of the washing effluent, resulting in the slower reduction rate in the 20–80 cm depth soils.
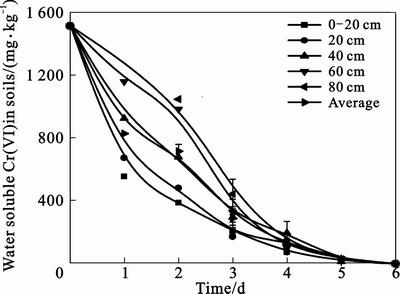
Fig. 5 Cr(VI) concentration change in soils with different depths
3.5 Effect of circulation mode
Soils with particle size <2 cm was packed into the columns and two kinds of process systems were employed to perform the bioremediation: System I, sprinkled and circulated with nutrient media till the bioremediation was finished. System II, sprinkled with tap water, adding nutrient media to the first day’s leachate to create suitable culture for bacteria washing down into the collector by the water from soils, and this was set as (i). So did the second day’s leachate, and this was set as (ii). When no chromium was detected in (i), substitute (i) for tap water to conduct the circulation until (ii) was ready to take over. Then, repeat the process till the soils were remediated.
As shown in fig. 6, table 7 and Table 8, both kinds of process system can completely remediate the chromium contaminated soils. And the remediated soil can be used as concrete aggregate and roadbed material according to Environmental protection technical specifications for pollution treatment of the Chromium residue [22] (table 8). The decrease of total chromium in soil may be because of the viscosity of the media which made microorganisms well adhere to the contaminated soils. Thus, Cr(VI) can be leached out and achieve reduction. What’s more, the remediated soils of bioprocess II had much more decrement of total Cr (VI) than I (table 7), though the time needed for completely bioremediation was longer (fig. 6). This illustrated that bioprocess II would better remediated the contaminated soil, for it favored the precipitating of Cr(OH)3 in the leachate collector, avoiding Cr(OH)3 mixed with soils, otherwise hindering the remediation process.
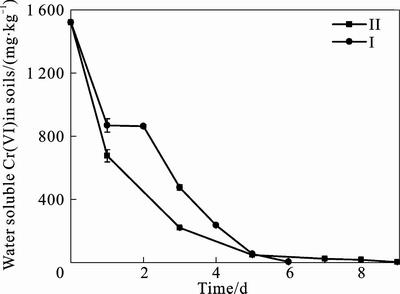
Fig. 6 Cr(VI) concentration change in soils with different techniques
Table 7 changes of different Cr species in soils after remediation with different techniques
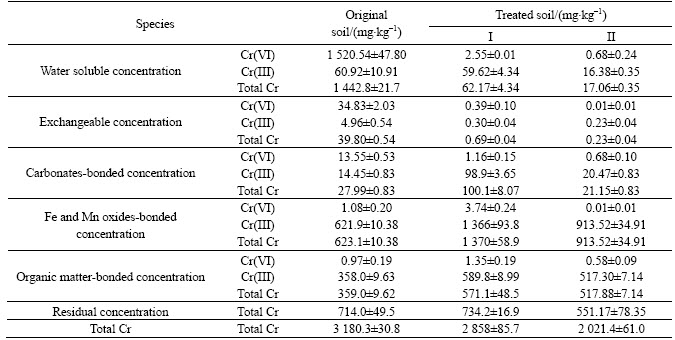
Table 8 Leaching toxicity of remediated soils with different techniques

4 Conclusions
1) The addition of nutrient media activates indigenous bacteria in soils and more than 99% water soluble Cr(VI), 90% exchangeable Cr(VI), 95% carbonates-bonded Cr(VI) are removed.
2) Optimum technique parameters are: particle size of the soils is less than 2 cm; spray intensity is 29.6–59.2 mL/min; time required for Cr(VI) detoxification is 6 d. the lower the initial Cr(VI) concentration, the higher removal rate the water soluble Cr(VI). the multiple spray circulation mode is superior to the single spray circulation system, and surface soils reduce fast than the subsoil.
3) Soils remediated could well fulfill the requirement of concrete aggregate and roadbed material usage.
References
[1] CHEUNG K H, GU Ji-dong. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review [J]. International Biodeterioration & Biodegradation, 2007, 59(1): 8–15.
[2] PATRA R C, MALIK S, BEER M, MEGHARAJ M, NAIDU R. Molecular characterization of chromium (VI) reducing potential in Gram positive bacteria isolated from contaminated sites [J]. Soil Biology and Biochemistry, 2010, 42(10): 1857–1863.
[3] COLIN V L, VILLEGAS L B, ABATE C M. Indigenous microoganisms as potential bioremediators for environments contaminated with heavy metals [J]. International Biodeterioration & Biodegradation, 2012, 69: 28–37.
[4] JEYASINGH J, PHILIP L. Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions [J]. Journal of Hazardous Materials, 2005, 118(1/2/3): 113–120.
[5] WANG Yun-yan, YANG Zhi-hui, CHAI Li-yuan, ZHAO Kun. Diffusion of hexavalent chromium in chromium-containing slag as affected by microbial detoxification [J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 1173–1178.
[6] KRISHNA K R, PHILIP L. Bioremediation of Cr(VI) in contaminated soils [J]. Journal of Hazardous Materials, 2005, 121(1/2/3): 109–117.
[7] MASOOD F, AHMAD M, ANSARI M A, MALIK A. Prediction of biosorption of total chromium by Bacillus sp. using artificial neural network [J]. Bull Environ Contam Toxicol, 2012, 88: 563–570.
[8] SHUKLA O P, RAI U N, DUBEY S. Involvement and interaction of microbial communities in the transformation and stabilization of chromium during the composting of tannery effluent treated biomass of Vallisneria spiralis L. [J]. Bioresource Technology, 2009, 100(7): 2198–2203.
[9] CHENG Guo-jun, LI Xiao-hua. Bioreduction of chromium (VI) by Bacillus sp. isolated from soils of iron mineral area [J]. European Journal of Soil Biology, 2009, 45(5/6): 483–487.
[10] SAHINKAYA E, ALTUN M, BEKTAS S, KOMNITSAS K. Bioreduction of Cr(VI) from acidic wastewaters in a sulfidogenic ABR[J]. Minerals Engineering, 2012, 32: 38–44.
[11] LEE S E, LEE J U,CHON H T, LEE J S. Reduction of Cr(VI) by indigenous bacteria in Cr-contaminated sediment under aerobic condition[J]. Journal of Geochemical Exploration, 2008, 96(2/3): 144–147.
[12] POOPAL A C, LAXMAN R S. Studies on biological reduction of chromate by Streptomyces griseus [J]. Journal of Hazardous Materials, 2009, 169(1/2/3): 539–545.
[13] MARTINS M, FALEIRO M L, CHAVES S, TENREIRO R. Anaerobic bio-removal of uranium (VI) and chromium (VI): Comparison of microbial community structure [J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 1065–1072.
[14] SHASHIDHAR T, PHILIP L, BHALLAMUDI S M. Bench-scale column experiments to study the containment of Cr(VI) in confined aquifers by bio-transformation[J]. Journal of Hazardous Materials, 2006, 131(1/2/3): 200–209.
[15] UMRANIA V V. Bioremediation of toxic heavy metals using acidothermophilic autotrophes [J]. Bioresource Technology, 2006, 97(10): 1237–1242.
[16] JEYASINGH J, SOMASUNDARAM V, PHILIP L, BHALLAMUDI S M. Bioremediation of Cr(VI) contaminated soil/sludge: Experimental studies and development of a management model [J]. Chemical Engineering Journal, 2010, 160(2): 556–564.
[17] JASTIN S, MADONA L P, MRUDULA P, MJOYCE N, NATARAJAN C, AMITAVE M. Hexavalent chromium bioremoval through adaptation and consortia development from sukinda chromite mine isolates [J]. Industrial & Engineering Chemistry Research, 2012, 51(9): 3740–3749.
[18] CHAI Li-yuan, HUANG Shun-hong, YANG Zhi-hui, PENG Bing, HUANG Yan, CHEN Yue-hui. Cr (VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag[J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 516–522.
[19] TESSIER A, CAMPBELL P G C, BISSON M. Sequential extraction procedure for the speciation of particulate trace metals[J]. Analytical Chemistry, 1979, 51(7): 844–851.
[20] KAPOOR A, VIRARAGHAVAN T. Fungal biosorption-an alternative treatment option for heavy metal bearing wastewaters: a review [J]. Bioresource Technology, 1995, 53(3): 195–206.
[21] USA Environmental Protection Agency. Environment protection agency office of solid waste. hazardous waste characteristics scoping study [M]. Washington DC: USA Environmental Protection Agency, 1996: 2–14.
[22] HJ/T 301-2007. Environmental protection technical specifications for pollution treatment of the Chromium residue (on trial) [S]. (in Chinese)
[23] SIGUA G, ISENSEE A, SADEGHI A M. Influence of rainfall intensity and crop residue on leaching of atrazine through intact no-till soil cores[J]. Soil Science, 1993, 156(4): 225–232.
[24] GHEJU M, IOVI A. Kinetics of hexavalent chromium reduction by scrap iron[J]. Journal of Hazardous Materials, 2006, 135(1/2/3): 66–73.
(Edited by HE Yun-bin)
Foundation item: Project(50925417) supported by the National Funds for Distinguished Young Scientist, China; Project(50830301) supported by the Key Program of National Natural Science Foundation of China; Project(51074191) supported by the National Natural Science Foundation of China
Received date: 2012–01–13; Accepted date: 2012–05–15
Corresponding author: YANG Zhi-hui; Professor; Tel: +86–731–88830875; Fax: +86–731–88710171; E-mail: yangzh@csu.edu.cn
Abstract: Bench-scale soil column experiments were carried out to evaluate the effectiveness of Cr(VI) bioremediation process in soils by using indigenous bacteria with the addition of bacteria nutrient media. Effects of particle size, spray intensity, initial Cr(VI) concentration, circulation mode and soil depth on Cr(VI) remediation were studied. Results show that soils after 6 d remediation with spray intensity controlled in the range of 29.6–59.2 mL/min could well fulfill the requirement of concrete aggregate and roadbed material usage, for the leaching toxicity concentration of the Cr(VI) in treated soils under the chosen condition is far less than 5 mg/L. The leaching toxicity and fractions of both hexavalent chromium and trivalent chromium from remediated soils were determined and compared with that of untreated soil. The results show that water soluble Cr(VI) declines from 1520.54 mg/kg to 0.68 mg/kg, exchangeable Cr(VI) decreases from 34.83 mg/kg to 0.01 mg/kg and carbonates-bonded Cr(VI) falls from 13.55 mg/kg to 0.68 mg/kg. Meanwhile, a corresponding increase in carbonate-bonded Cr(III), Fe and Mn oxides-bonded Cr(III) and organic matter-bonded Cr(III) are found. It reveals that indigenous bacteria can leach out water soluble Cr(VI), exchangeable Cr(VI) and carbonates-bonded Cr(VI) from contaminated soil followed by converting into carbonate-bonded Cr(III), Fe and Mn oxides-bonded Cr(III), organic matter-bonded Cr(III) and residual Cr(III).


