
Preparation and dielectric properties of porous silicon nitride ceramics
LI Jun-qi(李军奇), LUO Fa(罗 发), ZHU Dong-mei(朱冬梅), ZHOU Wan-cheng(周万城)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Porous silicon nitride ceramics with difference volume fractions of porosity from 34.1% to 59.2% were produced by adding different amount of the pore-forming agent into initial silicon nitride powder. The microwave dielectric property of these ceramics at a frequency of 9.36 GHz was studied. The crystalline phases of the samples were determined by X-ray diffraction analysis. The influence of porosity on the dielectric properties was evaluated. The results show that a-Si3N4 crystalline phase exists in all the samples while the main crystalline phase of the samples is b-Si3N4, indicating that the a/b transformation happens during the preparation of samples and the transformation is incomplete. There is a dense matrix containing large pores and cavities with needle-shaped and flaky β-Si3N4 grains distributing. The dielectric constant of the ceramics reduces with the increase of porosity.
Key words:
silicon nitride; pore-forming agent; porosity; geometry; dielectric properties;
1 Introduction
Silicon nitride ceramics have been recognized as one of the most important materials for high temperature applications. High temperature mechanical strength[1-3], good resistance to corrosion, wears and chemicals, low dielectric constant and loss, and super hardness with low density of silicon nitride[4-10] indicate a very optimistic future for high temperature radome applications[11].
The present study is concerned about the pressureless sintering of silicon nitride ceramics using Y2O3 and Al2O3 as sintering aid. Benzoic acid is added as pore-forming agent in order to allow the samples with different levels of porosity. The crystalline phase has been identified by XRD. Pore size and geometry have been measured for certain compositions using SEM. The effects of the porosity on the dielectric properties of the ceramics are studied.
2 Experimental
Silicon nitride powder (main size of 0.5 μm, α phase >96%) employed in this study was commercially available materials. Benzoic acid (chemically pure) was added as pore-forming agent. Y2O3 (99.99% purity) and Al2O3 (chemically pure) were used as sintering aid.
Silicon nitride powder with 5% Y2O3, and 5% Al2O3 as sintering aids was ball-milled in ethanol for 24 h. After the slurry had been dried in evaporator, the pore-forming agent was added at room temperature in varying volume of 10% to 40%. The resulting powder mixtures were uniaxially pressed at 100 MPa into disks measuring d80 mm×5 mm. The pressed green bodies were placed in an oven to decompose the pore-forming agents. The obtained porous green bodies were sintered in nitrogen at ambient pressure and at 1 800 ℃ for 1 h.
The bulk density and porosity of the sintered samples were measured by the ARCHIMEDE’s displacement method. The microstructures were characterized by scanning electron microscopy(S-570, Hitachi). Crystalline phases were identified by X-ray diffractometry(RINT 2000, Rigaku Co, Ltd). The samples were machined into d(61±0.1) mm×3 mm to measure the complex permittivity by the method of resonant cavity at a frequency of 9.36 GHz.
3 Results and discussion
A surface layer of silica surrounds every powder particle of the initial α-Si3N4. During firing, the Al2O3/ Y2O3 sintering aid reacts with the silica and some of the nitride forms an oxynitride liquid at high temperature, which promotes densification of the material. The oxynitride liquid exists as an intergranular glass phase at the grain boundaries when the ceramics cool. The role of the sintering aid[12, 13] is summarized by α-Si3N4+SiO2+Al2O3/Y2O3→b-Si3N4+Y-Si-Al-O-Nphase.
Fig.1 shows the XRD pattern of the sample. Only α-Si3N4 phase and b-Si3N4 phase are detected by X-ray diffraction analysis. b-Si3N4 phase content is about 80% for materials sintered at 1 800 ℃. At a given tempera- ture a large volume of liquid phase is formed and the α/β transformation occurs, which is beneficial for first-stage densification by particle rearrangement. Further densification is completed through solution-diffusion- reprecipitation. In this study, it is thought that the sintering took place in a non-oxidizing atmosphere produced by the use of a carbon crucible and a small amount of pore-forming agent remaining in the green body. In these circumstances, it is most likely that the liquid glassy phase (SiO2-Y2O3-Al2O3) decomposed at elevated temperatures to produce SiO gas. The SiO gas would continuously condense on the surface of the Si3N4 grain, resulting in the α/β transformation incompletely at 1 800 ℃.

Fig.1 XRD pattern of sample
The porous green bodies are prepared from the decomposition of pore-forming agent. Fig.2 shows the porosity of the samples prepared by the addition of pore-forming agent in varying volume fraction of 10% to 40%. The porosity of green bodies before sintering is an important parameter that determines the porosity of sintered samples. Under the same sintering condition, the samples show high porosity from 34.1% to 59.2% due to the difference volume fraction of porosity of green bodies resulting from pore-forming agent.
SEM micrographs of the porous silicon nitride prepared with 20% benzoic acid are shown in Fig.3. It shows two populations of pores: the smaller in the shape of spherical geometry of less than 1 mm, and the larger in irregular shape with dimensions up to approximately 6 mm. There are needle-shaped and flaky β-Si3N4 grains distributing in a dense matrix.

Fig.2 Relationship between porosity and volume fraction of pore-forming agent
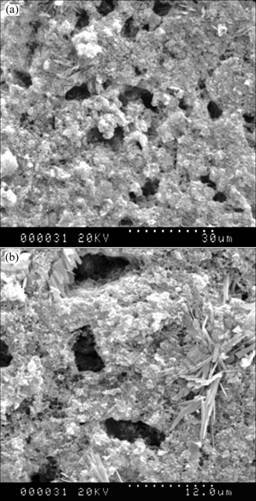
Fig.3 SEM micrographs of porous Si3N4 material with 20% benzoic acid
The dielectric properties of porous Si3N4 ceramics strongly depend on their porosity, microstructure and phase components. The effects of these elements on dielectric constant (e) could be characterized by mixture law[14] using the following expression:
![]() (1)
(1)
where φi is the volume fraction of i phase, εi is the dielectric constant of i phase. The dielectric constant decreases logarithmically with the increase of porosity.
Fig.4 shows the dielectric property as function of porosity of porous Si3N4 ceramics prepared by the pressureless sintering at 1 800 ℃ for 1 h. With an increase in porosity, the dielectric constant and dielectric loss (tan δ) of sintered samples decrease. The results show that the dielectric constant and dielectric loss decrease dramatically with rise of porosity, which is in accordance with the expression mentioned above. The lowest dielectric constant mainly results from the highest porosity; the highest dielectric constant and dielectric loss are 5.02 and 9.6?10-3 at a porosity of 34.1%.

Fig.4 Relationship between porosity and dielectric properties
4 Conclusions
Porous silicon nitride ceramics are fabricated using pore-forming agent, and the samples are produced with porosity from 34.1% to 59.2%. a-Si3N4 crystalline phase exists in the samples while the main crystalline phase of the samples is b-Si3N4, indicating that the a/b transformation happens during the preparation of samples and the transformation is incomplete. Microstructure consists of a dense matrix containing large pores and cavities with needle-shaped and flaky β-Si3N4 grains distributing in it. The dielectric constant of the ceramics decreases as the porosity increases.
References[1] AULT N N, YECKLEY R. Silicon nitride[J]. Amer Ceram Soc Bull, 1995, 74(6): 153-155.
[2] INAGAKI Y, KONDO N, OHJI T. High performance porous silicon nitride[J]. J Eur Ceram Soc, 2002, 22: 2489-2494.
[3] OHJI T, SHIGEGAKI Y, MIYAJIMA T, KANZAKI S. Fracture resistance behavior of multilayered silicon nitride[J]. J Am Ceram Soc, 1997, 80(2): 991-994.
[4] SHE J H, YANG J F, DANIEL D J, et al. Thermal shock behavior of isotropic and anisotropic porous silicon nitride[J]. J Am Ceram Soc, 2003, 86(4): 738-740.
[5] LEE S K, MORETTI J D, READEY M J, LAWN B R. Thermal shock resistance of silicon nitrides using an indentation-quench test[J]. J Am Ceram Soc, 2002, 85(1): 279-281.
[6] TOSHIMORI S. Shock synthesis of cubic silicon nitride[J]. J Am Ceram Soc, 2002, 85(1): 113-116.
[7] DENNIS S F, ELIZABETH J O, QUYNHGIAO N N, et al. Paralinear oxidation of silicon nitride in a water-vapor/oxygen environment[J]. J Am Ceram Soc, 2003, 86(8): 1256-1261.
[8] BACKHAUS-RICOULT M, GUERIN V, HUNTZ A M, URBANOVICH V S. High-temperature oxidation behavior of high-purity a, b, and mixed Silicon nitride ceramics[J]. J Am Ceram Soc, 2002, 85(2): 385-392.
[9] ZHANG Y, CHENG Y B, SRINIVASARAO L, et al. Erosion response of highly anisotropic silicon nitride[J]. J Am Ceram Soc, 2005, 88(1): 114-120.
[10] ANDREAS Z, MARKUS K, MARCUS S, et al. Elastic moduli and hardness of cubic silicon nitride[J]. J Am Ceram Soc, 2002, 85(1): 86-90.
[11] BARTA J, MANELA M, FISCHER R. Si3N4 and Si2N2O for high performance radomes[J]. Mater Sci Eng, 1985, 71: 265-272.
[12] HONMA T, UKYO Y. Sintering process of Si3N4 with Y2O3 and Al2O3 as sintering additives[J]. J Mater Sci Lett, 1999, 18: 735-737.
[13] YANG J F, OHJI T, NIIHARA K. Influence of yttria-alunina content on sintering behavior and microstructure of silicon nitride ceramics[J]. J Am Ceram Soc, 2000, 83(3): 2094-2096.
[14] KINGERY W D, BOWEN H K, UHLMANN D R. Introduction to Ceramics[M]. USA: John Wiley & Sons, 1976.
Foundation item: Project (50572090) supported by the National Natural Science Foundation of China
Corresponding author: LI Jun-qi; Tel: +86-29-88494574; Fax: +86-29-88494574; E-mail: junqlee@163.com


