盐酸头孢吡肟缓释微球的制备与工艺优化
彭东明1, 2,王春燕1,刘艳飞1, 3,李永欣1,苏小爱1,帅海涛1,盛毓1
1.中南大学 化学化工学院,湖南 长沙,410083;
2.湖南中医学院 药学院,湖南 长沙,410208;
3. 中南大学 有色金属资源化学教育部重点实验室,湖南 长沙,410083)
摘 要:
聚糖(OCMC)为载体、戊二醛(GA)为交联剂,采用乳化交联法制备包覆盐酸头孢吡肟(CD)的药物缓释微球。以OCMC质量分数、CD与OCMC质量比(m(CD)/m(OCMC))、交联剂用量(V(GA)/m(OCMC))及油相乳化剂Span80质量分数为自变量,以载药量和包封率为因变量,采用星点设计-效应面法优化制备工艺,并对优化后的工艺进行验证。所得最优制备条件如下:OCMC质量分数为2.7%,m(CD)/m(OCMC)为0.665,交联剂用量V(GA)/m(OCMC)为8 mL/g,Span80质量分数为4%;在最优工艺条件下制备的微球外观圆整,平均粒径为7 μm,粒度跨距为1.52,载药量和包封率分别为(21.4±0.5)%和(42.3±0.7)%,缓释时间达到10 d以上;星点设计-效应面法预测性良好。
关键词:
盐酸头孢吡肟;O-羧甲基壳聚糖;星点设计-效应面法;缓释微球;
中图分类号:O636.1 文献标志码:A 文章编号:1672-7207(2013)03-0907-07
Preparation and optimization of controlled release microspheres of cefepime dihydrochloride
PENG Dongming1, 2, WANG Chunyan1, LIU Yanfei1, 3, LI Yongxin1, SU Xiaoai1, SHUAI Haitao1, SHENG Yu1
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China;
3.Key Laboratory of Resources Chemistry of Nonferrous Metals,Ministry of Education,Central South University,Changsha 410083,China)
Abstract: O-carboxymethylchitosan (OCMC) microspheres containing an antibiotic drug cefepime dihydrochloride (CD) were prepared by emulsion crosslinking using glutaraldehyde (GA). The central composite design-response surface methodology was used to optimize process variables and the optimized formulation was validated. The OCMC concentration in the aqueous phase, mass ratio of CD to OCMC (m(CD)/m(OCMC)), the dosage of crosslinker (V(GA)/m(OCMC)) and the Span80 concentration in the organic phase were listed as independent variables and the dependent variables were drug-loading and encapsulation efficiency. The results show that the optimal values are as follows: the OCMC concentration is 2.7%, m(CD)/m(OCMC) is 0.665, the dosage of crosslinker (V(GA)/m(OCMC)) is 8 mL/g, the Span80 concentration is 4%. According to these conditions, the microspheres are spherical in shape. The mean diameter of the microspheres is 7 μm and the span is 1.52. The drug loading and drug encapsulation efficiency of CD-loaded microspheres are (21.4±0.5)% and (42.3±0.7)%, respectively. The microspheres are proved to be successful in prolonging drug release up to 10 d or more. The central composite design-response surface methodology has good prediction ability.
Key words: cefepime dihydrochloride; O-carboxymethylchitosan; central composite design-response surface methodology; controlled release microspheres
盐酸头孢吡肟(CD)是第四代头孢菌素,它对革兰氏阳性菌及革兰氏阴性菌有广谱抗菌性,且对革兰氏菌的抗菌活性优于第三代头孢菌素[1]。同时,与第三代头孢菌素相比,盐酸头孢吡肟拥有更好的耐受性[2]。但盐酸头孢吡肟的平均血浆消除半衰期只有2 h[3],临床上主要以增大剂量来延长给药间隔时间[4],这样不可避免地增加了机体内药物的血药浓度及药物对机体的毒性。尽管头孢吡肟的耐受性很好,但是,大剂量头孢吡肟仍存在毒性。有研究表明:头孢吡肟能产生神经毒素,可能会引起癫痫、紊乱、震颤、运动失调、躁动等不良反应[3]。把药物制成缓释长效制剂,可以使血药浓度平稳,避免谷峰现象,有利于减少药物的不良反应,提高患者的依从性,减少剂量等。O-羧甲基壳聚糖(OCMC)是壳聚糖的水溶性衍生物,它继承了壳聚糖优良的生物活性,同时改善了壳聚糖的水溶性,现已被广泛应用于控释缓释体系[5-7]。乳化交联法反应条件温和,有利于保持药物的结构和生物活性[8]。在此,本文作者选用乳化交联法,以戊二醛(GA)为交联剂,OCMC为载体制备了盐酸头孢吡肟O-羧甲基壳聚糖(CD-OCMC)缓释微球;采用星点设计-效应面法优化了制备工艺。
1 材料与方法
1.1 材料
O-羧甲基壳聚糖(青岛海普生物技术公司生产);25%(质量分数)戊二醛溶液(上海化学试剂公司生产);盐酸头孢吡肟(纯度为99.8%,上海新亚化工有限公司生产,储存于温度为4 ℃的冰箱中);其他试剂都为分析纯。
1.2 微球的制备
配制一定浓度的OCMC生理盐水溶液,常温下搅拌4 h至OCMC完全溶胀。加入一定量的CD,以转速50 r/min搅拌2 h。将上述混合均匀的溶液加入到50 mL包含一定量Span80的液体石蜡中,以转速500 r/min搅拌1 h。加入一定量的GA,交联固化2 h。砂芯漏斗抽滤,石油醚洗涤3次,丙酮洗涤3次,得浅黄色微球。在40 ℃的真空干燥箱中干燥得OCMC空白微球及CD-OCMC微球。
1.3 微球的性能及表征
采用激光粒度分布仪(MALVEN, MASTERSIZER 2000, British)测定微球的粒度分布情况;用光学显微镜(XSP-2C)及扫描电镜(SEM, JSM-6360)观察微球的形貌;对CD,OCMC,空白微球和载药微球进行红外光谱(FTIR)及X线衍射图谱(WXRD)测试。
1.4 标准曲线的绘制
精密称量10 mg CD 2份分别置于2个100 mL的容量瓶中,分别用pH=1.2的盐酸溶液、pH=7.3的磷酸缓冲溶液溶解并定容的测试液。取少测试液在200~500 nm波长范围内作紫外扫描。结果显示:2份测试液分别在257 nm及236 nm处有最强吸收。
取测试液0,0.2,0.5,1.0,2.0,3.0,5.0 mL置于25 mL的比色管中,加pH=1.2的盐酸溶液至刻度,于257 nm处测定CD标准液吸光度(A)。根据CD的浓度c和吸光度绘制标准曲线,对所得标准曲线进行线性回归,得出线性回归方程为:A=50.669 97c-0.001 05,R=0.999 94。以pH=7.3的磷酸缓冲溶液为溶剂的标准曲线回归方程为:A'=56.415 59c'-0.000 75,R=0.999 97。空白微球溶液在波长为257和236 nm处无紫外吸收峰,说明交联剂戊二醛对盐酸头孢吡肟的浓度检测无干扰。
1.5 微球载药量包封率的测定
精密称取20 mg载药微球,粉碎,加入到50 mL pH=1.2 HCl溶液中,在40 kHz超声3 h。取上清液用超滤膜(孔径为4.5 μm)过滤,使用紫外分光光度计(UV-9600)在257 nm处测定吸光度,由标准曲线计算出CD的浓度,载药量(Y1)及包封率(Y2)的计算公式如下:
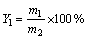 ,
,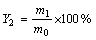
式中:m0为实际投药质量;m1为微球中药物质量;m2称取微球总质量。
1.6 星点设计优化制备工艺
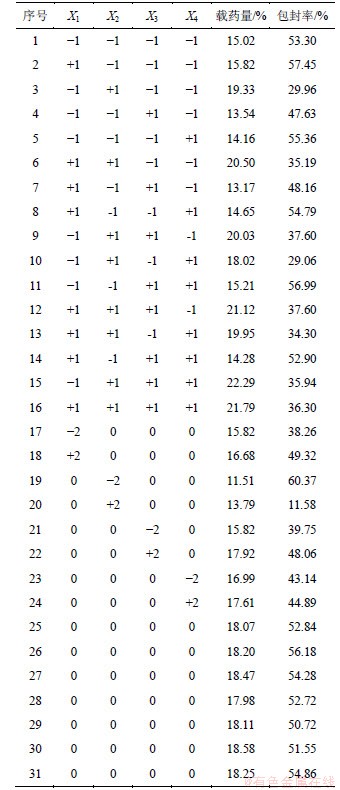
星点设计可以同时考察几个独立变量对因变量的影响,试验次数少、精确度高、应用方便[9-13]。在预实验的基础上,以OCMC质量分数(X1)、CD/OCMC质量比即m(CD)/m(OCMC)(X2)、交联剂用量(V(GA)/ m(OCMC),X3)及Span80质量分数(X4)为自变量,以Y1和Y2为因变量设计优化实验。各因素的水平根据预实验结果和实验需要设定,见表1。实验安排与结果如表2所示。
表1 因素水平表
Table 1 Factor levels for experimental design
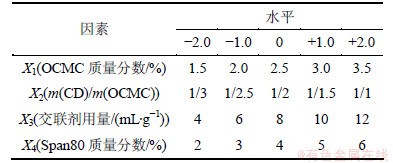
表2 星点设计与结果
Table 2 Central composite design and experimental results
1.7 体外释放
采用智能药物溶出仪(RCZ-8A)进行微球体外释放的性能研究。准确称取200 mg载药微球,置于透析袋中(截留相对分子质量为8 000~1 4000),再加入5 mL pH=7.3的磷酸缓冲溶液,扎紧两端,置于转篮子中。释放液为900 mL pH=7.3的磷酸缓冲溶液。调节温度为37 ℃,转速为100 r/min,隔一定时间取5 mL释放介质,在236 nm波长处测定紫外吸光度,同时补充释放液,由标准曲线计算累积溶出率。
2 结果与讨论
2.1 红外光谱分析
图1所示为OCMC、空白微球、载药微球、CD的红外光谱图。由图1可见:OCMC的特征吸收峰为3 428 cm-1处的O—H和N—H伸缩振动峰,1 618 cm-1处—COO-的反对称伸缩振动峰及N—H的伸缩振动峰,1 426 cm-1处的—COO-对称伸缩振动。交联后生成的空白微球新增了戊二醛的特征吸收峰(2 925 cm-1和2 854 cm-1处的C—CH2—C特征峰)。CD的特征吸收峰为2 400~3 550 cm-1处宽峰(羧酸离子,OH (H2O),NH2,CH3,CH2和CONH等的伸缩振动),1 773 cm-1处β-内酰胺环的伸缩振动,1 655.8 cm-1和1 630.5 cm-1处C=O和C=N的伸缩振动,1 544 cm-1和1 378 cm-1处羧酸盐的双吸收带,1 445.5 cm-1处CH2的剪式振动及CH3的不对称弯曲振动和1 046 cm-1处=C—H面内摇摆振动。载药微球的红外吸收光谱与空白微球的基本相同,新增的峰为微球中载入药物CD的特征吸收峰。CD的特征吸收峰(1 773 cm-1处β-内酰胺环的伸缩振动)出现在载药微球中。2 400~3 550,1 612,1 406,1 319及1 054 cm-1处的吸收峰强度增大,这归因于CD与空白微球的特征吸收峰的重叠。说明CD被成功包覆在OCMC中。
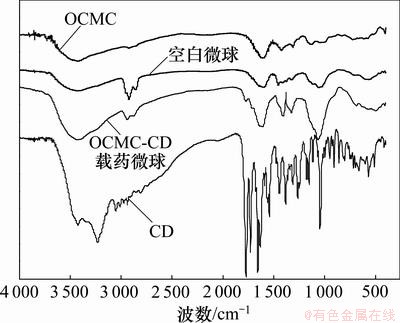
图1 OCMC、空白微球、载药微球、CD的红外光谱图
Fig.1 FTIR spectra of OCMC, placebo microspheres, CD-loaded microspheres and pure CD
2.2 X衍射图谱分析
CD,OCMC,空白微球和载药微球的X线衍射图谱如图2所示。可见:CD是一种结晶性的物质;OCMC为低结晶度物质;与OCMC相比,空白微球的结晶度下降,2θ=32°,44°的衍射峰消失,2θ=20°的峰强度减弱;载药微球的衍射图谱与空白微球的基本相似,载药微球中没有药物的结晶峰出现,说明药物以分子水平被包覆在载体中[14]。
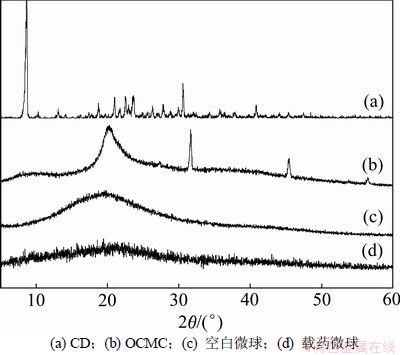
图2 CD,OCMC,空白微球和载药微球的X线衍射图谱
Fig.2 XRD spectra of CD, OCMC, placebo microspheres, and CD-loaded microspheres
2.3 微球的粒度与形貌分析
图3所示为空白微球和载药微球的光学显微镜图像,可见微球外观圆整,流动性好。图4所示为载药微球的SEM图,可以看出微球为光滑的球体,粒径小于10 μm。图5所示为载药微球的粒度分布图,可见载药微球粒径为0.36~19.96 μm,平均粒径为7 μm,粒度跨距为1.52,与SEM结果基本相符。
2.4 星点设计与效应面分析
采用SAS 9.2软件,以载药量和包封率为因变量,分别对各因素进行多元线性回归及二次多项式拟合。多元线性回归方程:
Y1=8.776+0.450X1+10.230X2+0.170X3+0.128X4
(显著性水平P=0.051 4, 相关系数R2 =0.295 0,
修正的相关系数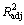 =0.186 6);
=0.186 6);
Y2=71.359+2.706X1-71.971X2+0.413X3+0.531X4
(P<0.000 1, R2=0.848 3,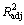 =0.825 0)。
=0.825 0)。
二次多项式方程:
Y1=-15.187+9.726X1+69.683X2-0.567X3-0.848X4-
1.528X12+3.370X1X2-70.698X22-0.319X1X3+2.377X2X3-

图3 空白微球与载药微球的光学显微镜图像
Fig.3 Light micrographs of placebo microspheres and CD-loaded microspheres
0.057X32- 0.213X1X4+0.119X2X4+0.300X3X4-0.119X42
(P<0.000 1, R2=0.975 8, 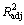 =0.954 7);
=0.954 7);
Y2=-55.430+5.532X1-69.187X2+6.207X3+2.114X4-
0.086X12+0.668X1X2-36.668X22-0.105X1X3+
7.031X2X3-0.530X32-0.011 8X1X4-7.949X2X4+
0.407X3X4-2.094X42
(P<0.000 1, R2=0.968 8, 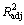 =0.941 4)。
=0.941 4)。
可见,二次多项式拟合效果优于多元线性回归方程。对二项式方程进行逐步回归分析,以达到简优化模型的目的[15-16]。
简化后的二次多项式方程:
Y1=-19.347+9.494X1+71.813X2-1.592X12+
3.370X1X2-71.389X22-0.356X1X3+2.272X2X3-
0.068X32+0.241X3X4-0.223X42
(P<0.000 1, R2=0.972 2, 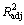 =0.958 3);
=0.958 3);
Y2=-65.368+54.083X1-52.476X2+7.836X3+
21.442X4-8.600X12-36.668X22-1.047X1X3+
7.031X2X3-0.530X32-7.949X2X4 -2.094X42
(P<0.000 1, R2=0.963 2, 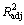 =0.941 9)。
=0.941 9)。
二次多项式方程简化前后回归系数的显著性水平(P)对比见表3。方程简化以后各回归系数的显著性水平明显提高。由简化后二次多项式方程的回归系数可见:各因素对载药量及包封率的影响由大到小为X2,X1,X4,X3。

图4 载药微球的SEM像
Fig.4 SEM images of CD-loaded microspheres
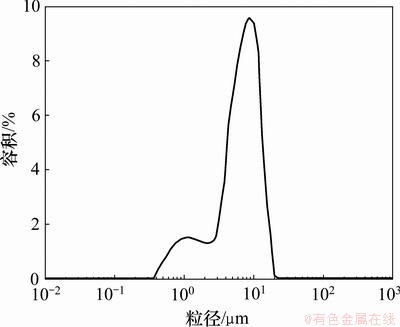
图5 载药微球的粒度分布
Fig.5 Particle size distribution of CD-loaded microspheres
根据简化的二次多项式方程,应用SAS 9.2软件分别绘制载药量和包封率与影响较大的2个因素X1和X2间的三维响应面图及等高线(其他自变量设为中点值),见图6。由图6(a)和6(b)可见:载药量随着OCMC质量分数(X1)的增大先增大后减小,当X1为2.8时,载药量最大;随着m(CD)/m(OCMC)(X2)的增加先增大后减小,当X2达0.76左右时,载药量最大。由预实验结果可知:本实验的关键在于配制均一的水相,但提高m(CD)/m(OCMC)不利于均一水相的配制。由图6(c)和6(d)可知:包封率随着OCMC质量分数(X1)的增大先增大后减小,当X1为2.7左右时,包封率最高;随着m(CD)/m(OCMC)(X2)的增大而减小,即CD/OCMC质量比越高,包封率越低。综合考虑各自变量对载药量和包封率的影响,得出最佳工艺参数为X1=2.7,X2=0.665,X3=8,X4=4。代入简化后的二次多项式方程,得载药量与包封率的预测值分别为21.1%和41.5%。
表3 回归系数的显著性水平
Table 3 Level significance of regression coefficient
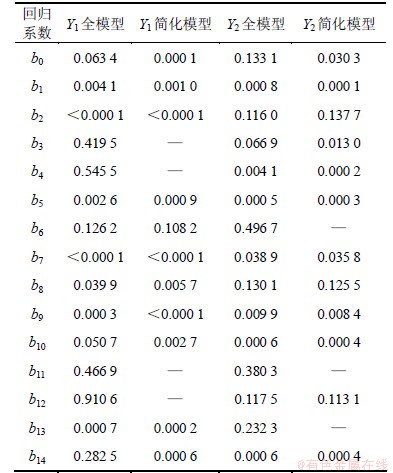
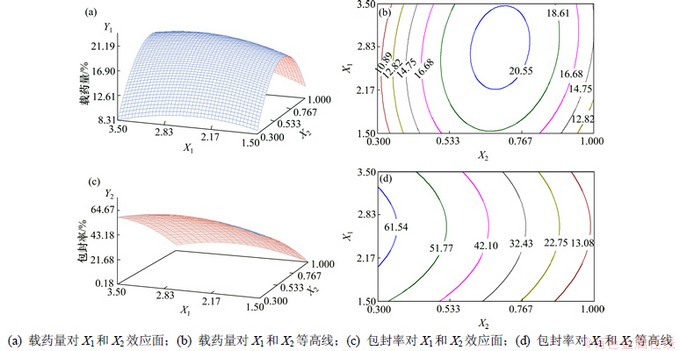
图6 因素X1和X2的效应面与等高线图
Fig.6 Response surface plots and contour lines of X1 and X2
在最优工艺参数下制备3批微球,测得微球载药量为(21.4±0.5)%,包封率为(42.3±0.7)%,预测值与实测平均值的偏差分别为1.42%和1.93%。说明简化后的二次多项式方程可以很好地预测因素与指标的关系。
2.5 微球的体外释放性能
图7所示为微球在pH=7.3磷酸缓冲溶液中的释放曲线图和直线拟合图。直线拟合结果表明:其相关性好。释药模型拟合表明微球在此缓冲液中的释放符合Higuchi方程:Qt=13.91+4.87t1/2(其中,Qt为不同时间的累积释放度;t为时间)。R=0.996,标准偏差Sd=2.168。
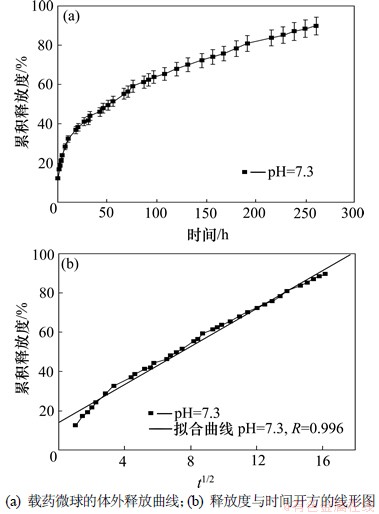
图7 载药微球的体外释放曲线及释放度与时间开方的线形图
Fig.7 Release profile of CD from CD-loaded microspheres and functional profile of square root of time and percentage of released CD
3 结论
(1) 采用乳化交联法,以戊二醛为交联剂,制备了包覆盐酸头孢吡肟的O-羧甲基壳聚糖微球。CD以分子水平被包覆在载体OCMC中。
(2) 根据星点设计,考察了OCMC水相质量分数、m(CD)/m(OCMC)、交联剂用量及油相乳化剂Span80质量分数对载药量与包封率的影响。通过多元线性拟合、二次多项式拟合、逐步回归及效应面分析,得出了最优工艺。在最优工艺条件下制备的微球为光滑的球体,平均粒径为7 μm,粒度跨距为1.52;体外释放表明其在pH=7.3的缓冲液中具有缓释作用;验证性实验表明:拟合方程能很好地预测因素与指标的关系,说明星点设计-效应面法预测性良好。
参考文献:
[1] Wynd M A, Paladino J A. Cefepime: A fourth-generation parenteral cephalosporin[J]. Ann Pharmac, 1996, 30 (12): 1414-1424.
[2] Neu H C. Safety of cefepime: A new extensed-spectrum parenteral cephalosporin[J]. Am J Med, 1996, 100(6A): 68S-75S.
[3] Bragatti J A. Cefepime-induced neurotoxicity[J]. Central Nervous System Agents in Medic Chem, 2008, 8: 229-233.
[4] 杨宝峰, 苏定冯, 周宏灏, 等. 药理学[M]. 北京: 人民卫生出版社, 2008: 23.
YANG Baofeng, SU Dingfeng, ZHOU Honghao, et al. Pharmacology[M]. Beijing: People’s Medical Publishing House, 2008: 23.
[5] ZHU Aiping, ZHANG Ming, ZHANG Zheng. Surface modification of ePTFE vascular grafts with O-carboxymethychitosan[J]. Polym Int, 2004, 53(1): 15-19.
[6] CAI Kaiyong, YAO Kangde, LI Zhi, et al. Rat osteoblast functions on the O-carboxymethy chitosan-modified poly(D,L-lactic acid) surface[J]. J Biomater Sci Polym Ed, 2001, 12(12): 1303-1315.
[7] ZHU Aiping, LIU Jianhong, YE Wenhui. Effective loading and controlled release of camptothecin by O-carboxymethychitosan aggregates[J]. Carbohydr Polym, 2006, 63(1): 89-96.
[8] 邓阳全, 邵丽, 杨银, 等. 乳化交联法在载药微球制备中的应用及研究进展[J]. 世界科技研究与发展, 2009, 31(1): 36-39.
DENG Yangquan, SHAO Li, YANG Yin, et al. Study on drug-carried microspheres prepared by emulsion cross-linking method[J]. World Sci-tech R&D, 2009, 31(1): 36-39.
[9] Zaghloul A A, Vaithiyalingam S R, Faltinek J, et al. Response surface methodology to obtain naproxen controlled-release tablets from its microspheres with eudragit L100-55[J]. J Microencapsulation, 2001, 18: 651-662.
[10] Hariharan M, Price J C. Solvent, emulsifier and drug concentration factors in poly(D,L-lactic acid) microspheres containing hexamethylamine[J]. J Microencapsulation, 2002, 19: 95-109.
[11] Abu-Izza K A, Garcia-Contreras L, Lu D R. Preparation and evaluation of sustained release AZT-loaded microspheres: Optimization of the release characteristics using response surface methodology[J]. J Pharm Sci, 1996, 85: 144-149.
[12] Gohel M C, Amin A F. Formulation optimization of controlled release diclofenac sodium microsheres using factorial design[J]. J Controlled Release, 1998, 51: 115-122.
[13] 刘艳杰, 项荣武. 星点设计效应面法在药学试验设计中的应用[J]. 中国现代应用药学杂, 2007, 24(6): 455-457.
LIU Yanjie, XIANG Rongwu. Application of central composite design-response surface methodology in pharmacy experiment design[J]. Chinese Journal of Modern Applied Pharmacy, 2007, 24(6): 455-457.
[14] Guyot M, Fawaz F. Nifedipine loaded-polymeric microspheres: preparation and physical characteristics[J]. Int J Pharm, 1998, 175: 61-74.
[15] Gohel M C, Amin A F. Formulation design and optimization of modified release microspheres of diclofenac sodium[J]. Drug Dev Ind Pharm, 1999, 25: 247-251.
[16] YUE Pengfei, ZHENG Qin, LIAO Meixiang, et al. Process optimization, characterization, and release study in vitro of an intravenous puerarion lipid micropheres loaded with the phospholipid complex[J]. J Dispersion Sci Techno, 2011, 32: 1-10.
(编辑 赵俊)
收稿日期:2012-04-17;修回日期:2012-06-20
基金项目:高等学校博士学科点专项科研基金资助项目(20090162120013);湖南省自然科学—株洲联合基金资助项目(12JJ9046);中南大学中央高校基本科研业务费专项资金项目(2012zzts053);国家大学生创新训练项目(AL120004);湖南省教育厅资助项目(11C0951)
通信作者:刘艳飞(1969-),女,湖南邵东人,博士,副教授,从事药物载体材料与制剂研究;电话:13017381167;E-mail: liuyf@ csu.edu.cn
摘要:以O-羧甲基壳聚糖(OCMC)为载体、戊二醛(GA)为交联剂,采用乳化交联法制备包覆盐酸头孢吡肟(CD)的药物缓释微球。以OCMC质量分数、CD与OCMC质量比(m(CD)/m(OCMC))、交联剂用量(V(GA)/m(OCMC))及油相乳化剂Span80质量分数为自变量,以载药量和包封率为因变量,采用星点设计-效应面法优化制备工艺,并对优化后的工艺进行验证。所得最优制备条件如下:OCMC质量分数为2.7%,m(CD)/m(OCMC)为0.665,交联剂用量V(GA)/m(OCMC)为8 mL/g,Span80质量分数为4%;在最优工艺条件下制备的微球外观圆整,平均粒径为7 μm,粒度跨距为1.52,载药量和包封率分别为(21.4±0.5)%和(42.3±0.7)%,缓释时间达到10 d以上;星点设计-效应面法预测性良好。


