
Tolerance and removal of chromium(Ⅵ) by
Bacillus sp. strain YB-1 isolated from electroplating sludge
LIU Yun-guo(刘云国), FENG Bao-ying(冯宝莹),
FAN Ting(樊 霆), ZHOU Hai-zhou(周海舟), LI Xin(李 欣)
College of Environmental Science and Engineering, Hunan University, Changsha 410082, China
Received 4 January 2007; accepted 10 October 2007
Abstract:
Four chromium(Ⅵ)-resistant bacteria named YB-1, YB-2, YB-3 and YB-4 were isolated from Cr-electroplating sludge. YB-1 and YB-2 were identified as a member of Bacillus sp. based on morphology and Biolog Microstation System. The strain of YB-1 was selected to test for its resistance and ability to remove Cr(Ⅵ) from aqueous solution. The results indicate that YB-1 exhibits high MIC value which can almost reach 140 mg/L and the growth of YB-1 in liquid medium containing 60 mg/L Cr(Ⅵ) is affected especially in the late exponential phase and stationary phase. Furthermore, the potential of living and freeze-dried YB-1 biomass to remove Cr(Ⅵ) was studied in different pH, biosorbent dose, contact time and initial concentration using the batch method. At the optimal conditions, living and freeze-dried biomass are capable of absorbing 34.5 mg/g and 17.8 mg/g chromium(Ⅵ) at initial concentration of 60 mg/L, respectively. The adsorption data were fitted to Langmuir isotherm model for these two sorbents. Kinetic studies show that the rates of sorption all follow the pseudo-second order kinetics.
Key words:
Bacillus sp.; Cr(Ⅵ); minimal inhibitory concentration; biosorption; kinetic model;
1 Introduction
Heavy metals like copper, lead, cadmium and chromium could cause serious threat to the environment and human health. Chromium is widespread used in leather tanning, electroplating, metal finishing and chromate preparation[1]. There are two common stable states of chromium, hexavalent chromium and trivalent chromium[2]. Hexavalent chromium species is present as either dichromate![]() in acidic environments or as chromate
in acidic environments or as chromate![]() in alkaline environments[3]. Chromium(Ⅵ) and its compounds are all water-soluble and mobile that can accumulate in human body throughout the food chain, therefore Cr(Ⅵ) is of particular concern and known to be much more harmful to human, animals and environment than Cr(Ⅲ). Cr(Ⅵ) has been recognized as one of the most dangerous heavy metals because it can cause skin or respiratory disease via diverse approaches and it is also carcinogenic. Consequently, removal of chromium(Ⅵ) from waste- water is quite essential.
in alkaline environments[3]. Chromium(Ⅵ) and its compounds are all water-soluble and mobile that can accumulate in human body throughout the food chain, therefore Cr(Ⅵ) is of particular concern and known to be much more harmful to human, animals and environment than Cr(Ⅲ). Cr(Ⅵ) has been recognized as one of the most dangerous heavy metals because it can cause skin or respiratory disease via diverse approaches and it is also carcinogenic. Consequently, removal of chromium(Ⅵ) from waste- water is quite essential.
Toxic metal ions are commonly removed by several conventional physicochemical methods which have been practiced for decades, such as precipitation, ion exchange, membrane separation, but certain drawbacks including high cost, low efficiency and operational complexity limit their usage.
Biological treatment using microorganisms or plants offers an alternative due to low operating costs of these materials and good performance[4]. Biosorption which is more effective especially at low metal concentration (below 100 mg/L) plays an important role in detoxification of metal pollution. Many microbial materials including bacteria, fungi and algae are considered having metal absorbing capacities. Adsorption of heavy metals was shown to be dependant on various factors like metal species, initial concentration, pH and contact time.
Many Cr-adsorption bacteria have been reported in previous researches[3-5]. Generally, biosorption of metals on bacteria commonly used growing or dead cells as biosorbent in previous studies. But removing heavy metals by living and freeze-dried bacteria cells is limited. Therefore, living and freeze-dried cells are applied in sorption of chromium(Ⅵ) in this work.
The objective of the present study is to screen anti- Cr(Ⅵ) bacterial isolates from chromium-contaminated site, test Cr(Ⅵ) adsorption capacity and compare the metal sorption behaviour of the living cells and freeze-dried cells. A Bacillus sp. strain YB-1 isolated from Cr-electroplating sludge is employed for removal of hexavalent chromium in this study. Various parameters affecting the sorption, such as initial concentration, biosorbents dose, pH of metal solution, and shaking time for each cell form are investigated. The sorption equilibrium and kinetic model are also studied.
2 Experimental
2.1 Isolation of Cr-resistant bacteria
The Cr-electroplating sludge sample was taken from Daxing Electroplate Factory in Changsha, China. It was collected in a sterile plastic bag and transported to the lab immediately. Bacteria were isolated on agar plates supplemented with different concentrations of Cr(Ⅵ) by dilution plate method as described[6]. The culture consisted of peptone 10 g, beef extract 5 g, NaCl 5 g and agar 20 g in 1 L deionized water and pH was adjusted to 7.0-7.2. The sample was serially diluted in 10-fold of 10-1-10-7 and 1 mL soil solution was dropped separately to the culture plates followed by pouring agar. The agar plates were incubated at 30 ℃ for 2 d. Colonies of distinct morphologies were selected to streak on separate plates and gradually enhance the concentration of Cr(Ⅵ) till there was no bacteria growing on the plates to obtain the Cr-resistant strains. Four pure isolates on 100 mg/L Cr(Ⅵ) culture were named strain YB-1, YB-2, YB-3 and YB-4. These isolates were purified and stored onto nutrient agar at 4 ℃.
Isolation and application of metal-resistant strains are greatly significant because high level of resistance maybe indicates potential ability of binding metals[7]. Generally, a microbe can be regarded as a chromium- resistant strain only if its tolerance level surpasses 10.4 mg/L which is the minimal inhibitory concentration (MIC) of E. coli[8].
2.2 Characterization of isolates
Preliminary characterization of the isolates was carried out by colony observations, microscope and Biolog Microstation System (Biolog, USA). Gram- staining and spore-staining were performed following conventional methods[6]. Bacteria were identified by Biolog Microstation System based on the differences of carbon source utilization.
2.3 Determination of MIC
Resistance of YB-1 to Cr(Ⅵ) was measured in liquid nutrient medium. 1 mL of YB-1 cells suspension (incubated for 24 h) was brought into 99 mL medium amended with different concentrations of Cr(VI) ranging from 0 to 200 mg/L. After another 24 h of incubation on a rotary shaker (150 r/min), the optical density(OD) value at 600 nm (OD600) of the medium was measured spectrophotometrically to monitor the growth of YB-1. Liquid medium in the absence of Cr(Ⅵ) served as the control. Minimal inhibitory concentration(MIC) is the lowest concentration of chromium that totally inhibits the growth of bacteria.
2.4 Effect of Cr(Ⅵ) on YB-1 growth
The effect of Cr(Ⅵ) on the growth of YB-1 was evaluated. YB-1 was cultivated in conical flasks containing growth medium with or without Cr(Ⅵ) (60 mg/L) on a rotary shaker (150 r/min) at 30 ℃ for 28 h, during which the OD600 of the medium was measured to determine the bacterial growth. Different sensitivity to Cr(Ⅵ) of each growth phase was investigated by comparing with these two corresponding bacterial growth curves (with and without metals).
2.5 Biosorbent preparation
YB-1 was cultivated on a rotary shaker (150 r/min) at 30 ℃. The cells in the exponential phase were harvested by means of centrifugation at 5 000 r/min for 5 min and washed several times with deionized water. The living cells were freeze-dried for 12 h and then marked as the freeze-dried cells in this work. The living cells and freeze-dried cells for further sorption experiment were all stored at 4 ℃ until use.
2.6 Biosorption experiment
The test solutions of required concentrations of chromium(Ⅵ) used in this study were prepared from analytical grade K2Cr2O7. The pH of each working solution was adjusted to the desirable value with 1 mol/L NaOH and 1 mol/L HCl.
The experiments were all conducted in 250 mL conical flask containing 100 mL metal solution on a rotary shaker, operating at 150 r/min after mixing the living or freeze-dried cells. Equal biomass was added to contact with 100 mL metal solution without nutriment and corresponding dry mass (0.1g) of living cells was calculated at each batch by drying an aliquot at 105 ℃ for 2 h. The effects of pH, initial concentration(C0), biosorbent dose(m) and contact time(t) were elucidated. Moreover, the adsorption equilibrium and kinetics were also studied in this work. Without special condition stated, pH, the initial concentration, contact time, temperature, quantity of the biomass and agitation rate were 2.0, 60 mg/L, 48 h, 30 ℃, 0.1 g dry mass (for both living and freeze-dried sorbents) and 150 r/min, respectively. And the solution pH was measured at different contact time.
The samples were centrifuged at 5 000 r/min for 5 min. Analysis of the residual chromium(Ⅵ) in the supernatant solution was operated by UV 754N model spectrophotometer at 540 nm using 1,5-diphenyl carbazide as the complexing agent in acidic solution. Each set of experiments was conducted in duplicate and average values were applied for further analysis.
The amount of Cr(Ⅵ) removed by these two kinds of biosorbent was calculated from the following equations:
![]() (1)
(1)
![]() (2)
(2)
where Q is the amount of absorbed metal ions per unit mass of sorbent (mg/g), R is the removal efficiency (%), C0 is the initial concentration of Cr(Ⅵ) (mg/L), Ce is the final concentration of Cr(Ⅵ) (mg/L), m is the dry mass of biomass (g), and V is the volume of the adsorption medium (L).
3 Results and discussion
3.1 Characterization of isolates
Colony, microscopic observation and Biolog results of these four isolates after 48 h of incubation on agar plates are listed in Table 1. YB-1 and YB-2 with endogenetic-spore are all Gram-positive and rod-shaped. YB-3 and YB-4 are Gram-negative but could not be identified by Biolog. The Biolog profiles gave YB-1 and YB-2 the same Genus ID: Bacillus. This result accords with the characterization of bacteria after consulting Bergey’s Manual of Determinative Bacteriology. For YB-1, SIM (Similarity) in Biolog to Bacillus mycoides is 0.44, SIM to Bacillus cereus or Bacillus thuringiensis is 0.09. Species listed in Biolog profiles all belong to Bacillus sp., but are significantly different from the isolates. This may imply that Bacillus sp. has become dominant in chromium-plating sludge. Bacillus sp. is considered as a kind of bacteria capable of accumulating a great deal of metals because of peptidoglycan on the cells wall[9]. YB-1 was selected to apply in the further resistance and sorption experiment. The studied strain YB-1 was incubated on agar plates and slants at 30 ℃ for 2 d and after that stored at 4 ℃.
Table 1 Characteristics and identification of isolates

There are numerous bacteria reported that resist heavy metals including Pseudomona sp.[10-12], Aeromona sp.[10], Bacillus sp.[3], etc.
3.2 Determination of MIC
The effect of different concentrations of Cr(Ⅵ) on the bacterial growth of cells is shown in Fig.1.
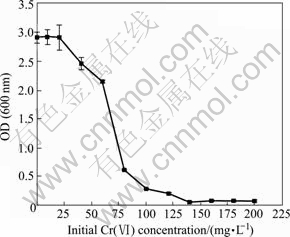
Fig.1 Effect of Cr(Ⅵ) concentration on growth of YB-1 (30 ℃, 150 r/min)
It is found that the growth of Bacillus YB-1 can not be affected at all when the concentration of Cr(Ⅵ) is below 25 mg/L. And its minimal inhibitory concentration (MIC) reaches 140 mg/L when no colony appears after streaking on the agar plates. These values indicate that YB-1 has formed its own resistance mechanism to Cr(VI) due to growing in Cr(Ⅵ)-contaminated site chronically, such as biosorption, accumulation, precipitation, reduction of Cr(Ⅵ) to Cr(Ⅲ), and chromate efflux[13].
3.3 Effect of Cr(Ⅵ) on YB-1 growth
The response of YB-1 growth to Cr(Ⅵ) is shown in Fig.2. The sensitivity to 60 mg/L Cr(Ⅵ) is various in different growth phases. In the lag and initial exponential growth phase, the growth of YB-1 can not be inhibited by Cr(Ⅵ) any more. But the bacterial biomass in Cr-containing medium decreases obviously in the middle, late exponential phase and the stationary phase compared with the control. Growth without metals shows a much longer exponential phase than that in the presence of 60 mg/L Cr(Ⅵ).
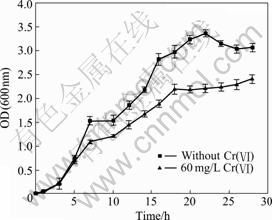
Fig.2 Growth of YB-1 with or without Cr(Ⅵ) (30 ℃, 150 r/min)
3.4 Effect of pH on Cr(Ⅵ) sorption
The influence of pH was tested by varying the pH in the range of 1.0 to 6.0. It was adjusted using 1 mol/L NaOH and 1 mol/L HCl when biomass had been added to the solution to avoid the effect of water in cells on pH.
The maximum metal uptake 25.5 mg/g (living cells) and 18.3 mg/g (freeze-dried cells) were all observed at pH 2.0 as shown in Fig.3. As the pH increases or decreases from 2.0, sorption behaviors are all inhibited more or less. It can be seen that the removal of Cr(Ⅵ) on living YB-1 is higher than that on freeze-dried cells at any given pH. Optimum pH 2.0 has been reported for removal of Cr(Ⅵ) by various materials[4,14-16].
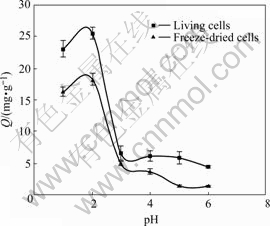
Fig.3 Effect of pH on Cr(Ⅵ) sorption (30 ℃, 150 r/min, m=0.1 g, C0=60 mg/L, t=48 h)
The pH can remarkably affect the chemical property of the functional groups (such as carboxylate, phosphate and amino groups) on the cells wall and metal ions in the solution. Therefore, adsorption of metal ions is pH dependent[17]. For other positive metal ions, low pH brings large hydrogen ions to compete with metal ions at binding sites resulting in low uptake, so that the optimum pH was usually between 5 and 9[18]. But Cr(Ⅵ) sorption is more complicated because chromium(Ⅵ) exists predominantly in the form of negative dichromate ions (such as ![]()
![]() in solution. The mechanism of effects of pH on sorption is that low pH (2.0) makes the surface of cells protonated which is propitious to anionic adsorption, leading to descending of Cr(Ⅵ) in solution. Cr(Ⅵ) sorption capacity also decreases as pH decreases to 1.0. This could be explained by TEWARI’s investigation that
in solution. The mechanism of effects of pH on sorption is that low pH (2.0) makes the surface of cells protonated which is propitious to anionic adsorption, leading to descending of Cr(Ⅵ) in solution. Cr(Ⅵ) sorption capacity also decreases as pH decreases to 1.0. This could be explained by TEWARI’s investigation that ![]() and
and ![]() are two comparatively active forms of Cr(Ⅵ) in contrast to
are two comparatively active forms of Cr(Ⅵ) in contrast to ![]() which is the dominant form at pH 1.0[16].
which is the dominant form at pH 1.0[16].
3.5 Effect of initial concentration on Cr(Ⅵ) sorption
The removal of Cr(Ⅵ) by living and freeze-dried cells of YB-1 is presented as a function of the initial concentration of Cr(Ⅵ) at pH 2.0. Fig.4 indicates that as the initial concentration increases from 40 mg/L to 400 mg/L, the sorption capacity increases from 22.3 mg/g to 79.5 mg/g for living biomass and 14.9 mg/g to 47.5 mg/g for freeze-dried one due to greater impetus. The maximum uptake 79.5 mg/g and 47.5 mg/g could be considered promising.
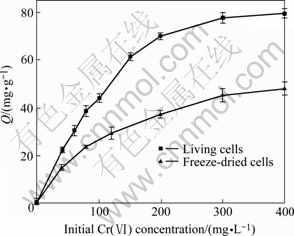
Fig.4 Effect of initial concentration on Cr(Ⅵ) sorption (30 ℃, 150 r/min, m=0.1 g, pH=2.0, t=48 h)
3.6 Effect of biosorbent dose on Cr(Ⅵ) sorption
The removal of chromium(Ⅵ) was evaluated by varying sorbent dosage from 0.01 g to 0.4 g (dry mass) at initial concentration of 60 mg/L. Quantity(Q) and efficiency(R) are plotted against the dosage of living and freeze-dried cells added to solution as presented in Fig.5.

Fig.5 Effect of biosorbent dose on Cr(Ⅵ) sorption (30 ℃, 150 r/min; C0= 60 mg/L, pH=2.0, t=48 h)
Reducing biomass means relatively elevating Cr(Ⅵ) concentration that uptake increases if only the biosorbent has not been saturated by ions. But R value increases because of more binding site supplied by more biosorbents. The sorption capacity is 45 mg/g and 28.21 mg/g when the mass of cells is just 0.01 g (dry mass) for living and freeze-dried cells, respectively.
3.7 Time course of biosorption
It can be seen from Fig.6 that the sorption capacity of Cr(Ⅵ) by living YB-1 increases rapidly within first 6 h (almost 50% of uptake at equilibrium) and does not change with time prolonging after 5 d. But equilibrium is established within 20 h for freeze-dried cells as presented in Fig.7.
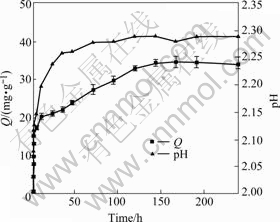
Fig.6 Time course and change of pH of biosorption on living biomass (30 ℃, 150 r/min, m=0.1 g, C0=60 mg/L, pH=2.0)

Fig.7 Time course and change of pH of biosorption on freeze-dried biomass (30 ℃, 150 r/min, m=0.1 g, C0=60 mg/L, pH=2.0)
During the course of sorption on living cells, we can observe the change of pH from initial pH 2.0 to 2.29 after 120 h of contact time as given in Fig.6 and a decrease of oxidation reduction potential as removal of Cr(Ⅵ). The pH increases obviously just in the first 6 h and slowly after that, which is very similar to the removal rate of Cr(Ⅵ). PARK et al also reported that the solution pH increased abruptly from 2.00 to 2.13 after 158 h in Cr(Ⅵ) biosorption experiment using dead Aspergillus. niger[19]. But no increase of pH was observed in the course of sorption on freeze-dried YB-1.
As we know, the reduction of Cr(Ⅵ) to Cr(Ⅲ) requires electron donors, resulting in a decrease of the oxidation reduction potential corresponding to the increase of pH. The increase of pH can be explained by the following reaction: ![]() +14H+ +6e→2Cr3++ 7H2O[20]. All these phenomena predict that Cr(Ⅵ) may be reduced to comparatively innocuous Cr(Ⅲ) by living cells without extra electron donors. In some cases, intracellular enzymes contribute to Cr(Ⅵ) reduction[21]. Special enzymes may exist in living Cr-resistant YB-1 due to high concentration chromium condition it grew in before. As a result of redox reaction, more Cr(Ⅵ) is removed from solutions by living cells than freeze-dried cells in above-mentioned tests. That’s why isolation from contaminated sites could provide preponderant strains helpful to removing pollution. Much work remains to be done about the reduction of Cr(Ⅵ) by YB-1 in the future.
+14H+ +6e→2Cr3++ 7H2O[20]. All these phenomena predict that Cr(Ⅵ) may be reduced to comparatively innocuous Cr(Ⅲ) by living cells without extra electron donors. In some cases, intracellular enzymes contribute to Cr(Ⅵ) reduction[21]. Special enzymes may exist in living Cr-resistant YB-1 due to high concentration chromium condition it grew in before. As a result of redox reaction, more Cr(Ⅵ) is removed from solutions by living cells than freeze-dried cells in above-mentioned tests. That’s why isolation from contaminated sites could provide preponderant strains helpful to removing pollution. Much work remains to be done about the reduction of Cr(Ⅵ) by YB-1 in the future.
3.8 Equilibrium study
Langmuir and Freundlich isotherm models were widely used in the biosorption literature.
The Langmuir isotherm is valid for monolayer sorption and it could be described as follows:
![]() (3)
(3)
and the linearized form of Eqn.(3) is
![]() (4)
(4)
where Qeq (mg/g) and Ceq (mg/L) are the uptake of sorption and the residual concentration at equilibrium. Qmax and b are the adsorption isotherm parameters. Ceq/Qeq vs Ceq was plotted (Fig.8), Qmax and b can be calculated from the slop and the intercept.

Fig.8 Langmuir adsorption isotherm of Cr(Ⅵ) on YB-1 (30 ℃, 150 r/min, m=0.1 g, pH=2.0)
The Freundlich equation is empirical and it is presented as
![]() (5)
(5)
The logarithmic form of Eqn.(5) is
![]() (6)
(6)
where KF and n are the Freundlich constant and exponent. ln Qeq vs ln Ceq is plotted (Fig.9), n and KF can be determined from the slop and the intercept.
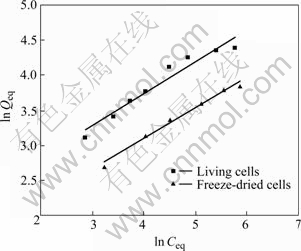
Fig.9 Freundlich adsorption isotherm of Cr(Ⅵ) on YB-1 (30 ℃, 150 r/min, m=0.1 g, pH=2.0)
The parameters of Langmuir and Freundlich model obtained at 30 ℃ are presented in Table 2. The adsorption data of Cr(Ⅵ) can be well described by Langmuir isotherm model given in Fig.8 with the coefficient (R2) of 0.995 5 for both two kinds of biosorbent. This illuminates that monolayer sorption is dominant for Cr(Ⅵ) sorbed on living and freeze-dried cells in this research. The magnitude of Kf and n shows easy separation of Cr(Ⅵ) from aqueous solution and high adsorption capacity[22].
Table 2 Adsorption isotherm parameters for Cr(Ⅵ)

3.9 Kinetic modeling
The pseudo-first order and pseudo-second order rate equation were used in kinetic study.
The first order rate equation is given as
![]() (7)
(7)
which can be expressed after integrating as below:
![]() (8)
(8)
where Qt and Qeq are sorption capacity at time t and at equilibrium, respectively. K1,ad is the pseudo-first order rate constant. lg(Qeq-Qt) vs t is plotted (Fig.10) and K1,ad can be determined from the slop.

Fig.10 Linearized form of pseudo-first order model (30 ℃, 150 r/min, m=0.1 g, C0=60 mg/L, pH=2.0)
The pseudo-second order rate equation is expressed as
![]() (9)
(9)
The integrated form of the pseudo-second order rate equation is commonly presented as
![]() (10)
(10)
where K2, ad is pseudo-second order rate constant. K2, ad can be calculated from the slop of the plot of 1/Qt vs 1/t (Fig.11).

Fig.11 Linearized form of pseudo-second order model (30 ℃, 150 r/min, m=0.1 g, C0=60 mg/L, pH=2.0)
The parameters of pseudo-first and pseudo-second order model are presented in Table 3. The rate of sorption on living and freeze-dried biomass all follows the pseudo-second order kinetics because the correlation coefficients of the second order was slightly higher than that of the first order. And the theoretical Qeq values of the pseudo-second order rate kinetics are also consistent with the experimental ones.
Table 3 First-order and second-order adsorption rate constants for biosorption of Cr(Ⅵ)

4 Conclusions
Four Cr-resistant strains were isolated and two of them were identified as a member of Bacillus sp. by Biolog. The isolate YB-1 exhibited tolerance to chromium(Ⅵ) and the minimal inhibitory concentration was 140 mg/L. The results of biosorption study demonstrated that both living and freeze-dried cells of chromium(Ⅵ)-resistant bacterium YB-1 could remove chromium(Ⅵ) from dilute solutions. Living cells and freeze-dried cells were capable of removing 34.5 mg/g and 17.8 mg/g chromium(Ⅵ) at optimum pH of 2.0, initial concentration of 60 mg/L. Biosorption equilibrium was finished after about 5 d for living cells, 20 h for freeze-dried cells, respectively. The adsorption data all fit to Langmuir isotherm model at 30 ℃. The variety of pH in the course of biosorption was measured and we observed an increase of pH from 2.0 to 2.29 after 120 h using living biomass. This finding indicated that the reduction of Cr(Ⅵ) may happen while chromium is bound to the living cells.
References
[1] SRIVASTAVA S, THAKUR I S. Isolation and process parameter optimization of Aspergillus sp. for removal of chromium from tannery effluent [J]. Bioresource Technol, 2006, 97: 1167-1173.
[2] DAKIKY M, KHAMI A, MANASSRA A, MER’EB M. Selective adsorption of chromium(Ⅵ) in industrial wastewater using low-cost abundantly available adsorbents [J]. Advances in Environmental Research, 2002, 6(4): 533-540.
[3] SRINATH T, VERMA T, RAMTEKE P W, GARG S K. Chromium(Ⅵ) biosorption and bioaccumulation by chromate resistant bacteria [J]. Chemosphere, 2002, 48: 427-435.
[4] SAHIN Y, OZTURK A. Biosorption of chromium(Ⅵ) ions from aqueous solution by the bacterium Bacillus thuringiensis [J]. Process Biochem, 2005, 40: 1895-1901.
[5] ZHOU Ming, LIU Yun-guo, LI Xin, XU Wei-hua, FAN Ting, NIU Yi-le. Kinetic studies on Cr6+ biosorption by Bacillus licheniformi [J]. Chin J Appl Environ Biol, 2006, 12: 84-87. (in Chinese)
[6] ZHOU Qun-ying, GAO Ting-yao. Microbiology of environmental engineering [M]. Beijing: Higher Education Press, 2001. (in Chinese)
[7] ZHANG Han-bo, ZHENG Yue, ZENG Fan, ZHU Zhi-ying, WANG Jie. Resistance and adsorption of several bacterial strains to heavy metal [J]. Microbiology, 2005, 32: 24-29. (in Chinese)
[8] PEI Yao-wen, LUO Zhu-hua, HUANG Xiang-ling, WANG Lin, YE De-zan. Isolation and identification of chromate-tolerant deep-sea bacteria and their chromate reduction capability [J]. Acta Oceanologica Sinica, 2004, 26: 142-148. (in Chinese)
[9] HUANG Shu-hui. The mechanism of binding metals by bacteria [J]. Microbiology, 1992, 19: 171-173 (in Chinese)
[10] LIN Yi, FANG Guang-wei, CAI Li-xi, PENG Kun. Screening and molecular identification for heavy metal-resistant isolate [J]. Journal of Quanzhou Normal University (Natural Science), 2005, 23: 73-76. (in Chinese).
[11] HUSSEIN H, FARAG S, KANDIL K, MOAWAD H. Tolerance and uptake of heavy metals by pseudomonads [J]. Process Biochem, 2005, 40: 955-961.
[12] RAJA C E, ANBAZHAGAN K, SELVAM G S. Isolation and characterization of a metal-resistant Pseudomonas aeruginosa strain [J]. World J Microb Biot, 2006, 22: 577-585.
[13] CERVANTES C, CAMPOS-GARCA J, DEVARS S, GUTI?RREZ- CORONA F, LOZA-TAVERA H, TORRES-CUZM?N J C, MORENO-SANCHEZ R. Interactions of chromium with microorganisms and plants [J]. FEMS Microbiology Reviews, 2001, 25: 335-347.
[14] AGARWAL G S, BHUPTAWAT H K, CHAUDHARI S. Biosorption of aqueous chromium(VI) by Tamarindus indica seeds [J]. Bioresource Technol, 2006, 97: 949-956.
[15] OZDEMIR G, OZTURK T, CEYHAN N, ISLER R, COSAR T. Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge [J]. Bioresource Technol, 2003, 90: 71-74.
[16] TEWARI N, VASUDEVAN P, GUHAB B K. Study on biosorption of Cr(Ⅵ) by Mucor hiemalis [J]. Biochem Eng J, 2005, 23: 185-192.
[17] SHI J G, YUAN X Z , ZHANG S F, DAI Fang. Removal of cadmium and lead from sediment by rhamnolipid [J]. Trans Nonferrous Met Soc China, 2004, 14(1): 66-70.
[18] WANG Jian-long, HAN Ying-jian, QIAN Yi. Advances in research on biosorption of metals [J]. Microbiology, 2000, 27: 449-452. (in Chinese)
[19] PARK D, YUN Y S, JO J H, PARK J M. Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger [J]. Water Res, 2005, 39: 533-540.
[20] XU Wei-hua, LIU Yun-guo, ZENG Guang-ming, LI Xin. TANG Chun-fang, YUAN Xin-Zhong. Enhancing effect of iron on chromate reduction Cellulomonas flavigena [J]. J Hazard Mater, 2005, 126: 17-22.
[21] KRISHNA K R, PHILIP L. Bioremediation of Cr(Ⅵ) in contaminated soils [J]. J Hazard Mater, 2005, 121: 109-117.
[22] LIU Yun-guo, FAN Ting, ZENG Guang-ming, LI Xin, TONG-qing, YE Fei, ZHOU Ming, XU Wei-hua, HUANG Yu-e. Removal of cadmium and zinc ions from aqueous solution by living Aspergillus niger [J]. Trans Nonferrous Met Soc China, 2006, 16: 681-686.
Foundation item: Project(04JJ3013) supported by the Natural Science Foundation of Hunan Province, China; Project(20050532009) supported by the Doctoral Fund of Ministry of Education of China
Corresponding author: LIU Yun-guo; Tel: +86-731-8649208; E-mail: Liuyunguo@hun.cn


