
Influence of CaO on damping capacity and mechanical properties of Mg alloy
Dong-In JANG1, Jin-Kyu LEE1, Dae-Up KIM2, Shae-K KIM1
1. Advanced Material Division, Korea Institute of Industrial Technology, Incheon, Korea;
2. Hyudai Mobis, 8-10 Mabuk-dong, Yongin, Gyunggi-do, 446-715, Korea
Received 2 March 2009; accepted 30 May 2009
Abstract:
For developing cost effective damping Mg alloys with high damping capacity and better mechanical properties, the microstructures, the mechanical properties at room temperature and the damping capacity of Mg alloy adding CaO were investigated and compared with those of K1A alloy. CaO adding into pure Mg maintains the damping capacity and increases the ultimate tensile strength compared with those of pure Mg. Mg-CaO alloy can be regarded as cost-effective damping alloy with high damping and mechanical properties as well as with the advantages of improving oxidation and burning resistances.
Key words:
Mg alloy; CaO; Mg-CaO; damping capacity;
1 Introduction
With development of modern industry and transportation, noise pollution has became one of the serious environmental problems[1]. The development and application of high damping materials is one of the effective method to reduce noise. Mg and its alloys are the lightest structural materials with high specific strength and high specific elastic modulus[2]. Pure Mg has the best damping capacity among various metallic materials. However, poor mechanical properties of pure Mg limit its more widespread applications. Introducing precipitates of a secondary phase that is able to pin the dislocations and increasing the yield stress can harden pure Mg. However, attention must be paid to choice the alloying elements if we want to maintain high damping capacity. The dislocation loops defined between the precipitates must be free to vibrate. The condition for this is that the Mg matrix has to be relatively pure. In other words, the solubility of the alloying elements in Mg matrix must be very low.
Mg-0.7%Zr (K1A) alloy has the best damping capacity among metallic materials. However, high cost and difficult alloying limit more widespread applications of Zr[3]. Mg-Ni alloys were reported to possess high damping capacities at room temperature[4-5]. And it was concluded that the very small solubility of Ni in Mg matrix and the shape of the primary Mg in the Mg-Ni alloys were responsible for the much lower critical strain than those of other Mg alloys and pure Mg. However, Mg-Ni is not an interesting system for damping application because of its poor corrosion resistance[6]. Thus, it is becoming a continuous subject to develop Mg alloys with high damping capacities.
CaO adding into pure Mg is considered a possible damping material. On the one hand, CaO adding into Mg alloys does not form solid solution while CaO is very cheap. On the other hand, CaO can also improve oxidation and ignition resistances of Mg alloys, which makes them possible to make SF6 gas free melting and casting process and ensure safety for Mg components [7-11]. For developing cost effective damping Mg alloys with high damping capacity and better mechanical properties[12], the microstructures and the mechanical properties at room temperature and the damping capacity of Mg-CaO alloy were investigated and compared with those of the K1A alloy.
2 Experimental
Pure Mg was melted in a steel crucible in an electric resistance furnace at 700 ℃ under SF6 and CO2 gases. The desired fraction of CaO was added into molten pure Mg. The CaO added melts were cast into steel mold preheated at 200 ℃. For optical examination, the samples
δ=ln(An/An+1) (1)
where An and An+1 represent the strain amplitude of the nth and (n+1)th cycles in free decay, respectively.
3 Results and discussion
Fig.1 shows the microstructures of pure Mg, Mg-Zr and Mg-CaO alloys in the as-cast condition. From Fig.1, the microstructures are evaluated on the edge and center of pure Mg, Mg-Zr and Mg-CaO alloys using the Olympus PME3 microscope. In pure Mg and Mg-CaO alloys, no difference exists. The microstructures are uniform in all the sections of edge and center in the both samples. However, in the case of Mg-Zr alloy, the microstructure is comparatively grain refined.
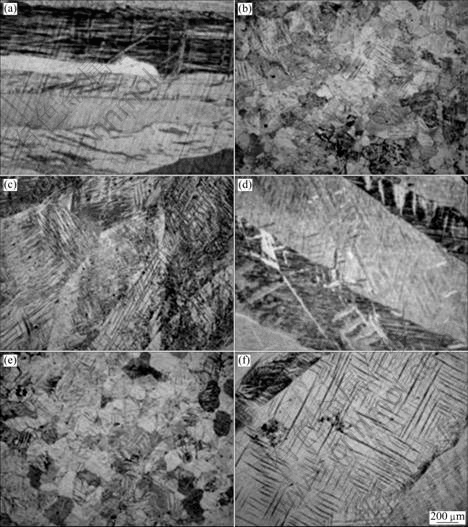
Fig.1 Microstructures of edge position and center position of pure Mg, Mg-Zr alloy and Mg-CaO alloys: (a) Pure Mg, edge; (b) Mg-Zr alloy, edge; (c) Mg-CaO alloy; (d) Pure Mg, center; (e) Mg-Zr alloy, center; (f) Mg-CaO alloy, center
Fig.2 shows the hardness values of pure Mg, Mg-Zr and Mg-CaO alloys in the as-cast condition. From Fig.2, hardness tests are conducted on the edge and center of pure Mg, Mg-Zr and Mg-CaO alloys using the Rockwell hardness tester. The Rockwell hardness values are the same for pure Mg and Mg-CaO alloys. On the other hand,the Rockwell hardness values of Mg-Zr alloy are little higher than those of pure Mg and Mg-CaO alloys due to the grain refinement achieved.
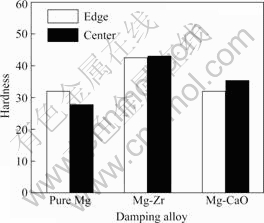
Fig.2 Hardness of pure Mg, Mg-Zr and Mg-CaO alloys
Fig.3 shows the mechanical properties of pure Mg, Mg-Zr and Mg-CaO alloys in the as-cast condition. The ultimate tensile strength and elongation of pure Mg are 54.12 MPa and 11.33%, respectively. The ultimate tensile strength and elongation of Mg-Zr alloy are 69.03 MPa and 8.3%, respectively. The ultimate tensile strength of Mg-Zr alloy is little bit higher than that of pure Mg. However, the elongation of Mg-Zr alloy is little smaller than that of pure Mg. On the other hand, the ultimate tensile strength and elongation of Mg-CaO alloy are 65.22 MPa and 11.87%, respectively. The ultimate tensile strength of Mg-CaO alloy is little brgger than that of pure Mg. The elongation of Mg-CaO alloy is similar to that of pure Mg. The ultimate tensile strength values obtained are much smaller than the reference data, because the tensile strength test is done for the as-gravity cast specimen with much porosity and inclusions inside them. However, the important point is that the ultimate tensile strength of Mg-CaO alloy is similar to that of Mg-Zr alloy, while the elongation of Mg-CaO alloy is little bigger than that of Mg-Zr alloy.
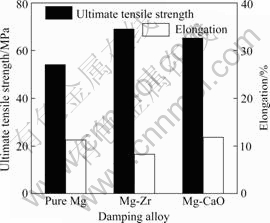
Fig.3 Mechanical properties of pure Mg, Mg-Zr alloy and Mg-CaO alloys
Fig.4 shows the Granato–Lücke model[13]. The damping mechanism of Mg or Mg alloys can be explained by Granato–Lücke model. For a small stress, the dislocation loops pinned down by the impurity particles bow out and continue to bow out until the breakaway stress is reached. At the breakaway stress, the dislocation strain increases without increase of stress. The loss caused by dislocation segment pinned by strong pinning points is named as static hysteresis internal friction, and is strain-amplitude dependent and frequency independent. Considering the mechanism, it is reasonable to expect that the bigger the strength, the smaller the damping capacity responsing to the increased breakaway stress.
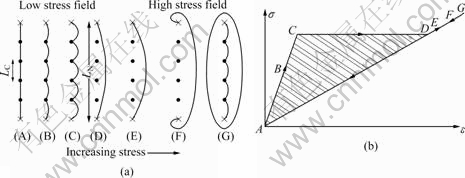
Fig.4 Granato–Lücke model: (a) Bowing out, breakaway and multiplication of dislocation by increasing applied stress; (b) Stress-dislocation strain law
Above results can be interpreted by using Granato–Lücke model[13] (Fig.4(a)). For large enough concentration of impurity atoms, the length of loop determined by the intersection of dislocation network loops is further pinned down by the impurity particles through the Cottrell mechanism. There are, therefore, two characteristic lengths in the model, namely, the network length LN and the loops length LC, determined by the impurities. If an external stress is applied, there will be, in addition the elastic strain, an additional strain due to the dislocations strain. For zero applied stress, the length LN is pinned down by the impurity particles (A). For a very small stress (B), the loops length (LC) bow out and continue to bow out until the breakaway stress is reached. At the breakaway stress, a large increase in the dislocation strain occurs without stress increasing (C–D). Now, for further increases in the stress, the network length (LN) bows out (D–E), until the stress required to activate the Frank–Read source[14], (LN) is reached. Further increasing the applied stress leads to creation and expansion of new closed dislocation loops (F–G). The stress—dislocation strain law corresponding to the model is shown in Fig.4(b). The curve has a hysteresis loop due to the different dislocation movement, which leads to the internal friction.
Fig.5 shows the tensile strength and specific damping capacities of pure Mg, Mg-Zr alloy and Mg-CaO alloy. The tensile strength and specific damping capacity of pure Mg are 54.12 MPa and 79.9%, respectively. The tensile strength and specific damping capacity of Mg-Zr alloy are 69.03 MPa and 82.4%, respectively. The specific damping capacity of pure Mg is similar to that of Mg-Zr alloy. The tensile strength and specific damping capacity of Mg-CaO alloy are 65.22 MPa and 86.7%, respectively. The tensile strength and specific damping capacity of Mg-CaO alloy are little bigger than those of pure Mg. While the tensile strength of Mg-CaO alloy is similar to that of Mg-Zr alloy, the specific damping capacity of Mg-CaO alloy is bigger than that of Mg-Zr alloy. CaO adding into pure Mg maintains the damping capacity and increases the ultimate tensile strength compared with those of pure Mg, which demonstrates the possibility of Mg-CaO alloy as cost effective damping material. Further study is now being carried out to optimize CaO composition and other added materials, such as Si and SiC.

Fig.5 Tensile strength and specific damping capacities of pure Mg, Mg-Zr and Mg-CaO alloys
4 Conclusions
1) Mg-Zr alloy has high specific damping capacity and improved hardness and tensile properties at room temperature. However, high cost and difficult-to-alloy aspects of Zr limit its widespread applications.
2) The damping capacity of Mg-CaO alloy was compared with that of Mg-Zr alloy. It is important that CaO adding into pure Mg maintains the damping capacity and increases the ultimate tensile strength compared with those of pure Mg. Further study is necessary to optimize CaO amount and evaluate the combination effect of other alloying elements for Mg damping alloys.
3) Mg-CaO alloy can be regarded as cost-effective damping alloy with high damping and mechanical properties as well as with the advantages of improving oxidation and burning resistances.
REFERENCES
[1] RUBHERA R, MATO A M, MUFURUKI T S. Noise pollution associated with the operation of the Dar es Salaam international airport [J]. Transportation Research Part D: Transport and Environment, 1999, 4(2): 81-89.
[2] AVEDESIAN M M, BAKER H. ASM international’s binary alloy phase diagrams (electronic version) [M]. 2nd ed. Ohio: ASM International Materials Park, 1999.
[3] ELEKTRON Z A. Datasheet No. 462Magnesium Elektron Ltd.
[4] SUGIMOTO K, NIIYA K, OKAMOTO T, KISHITAKE K. A study of damping capacity in magnesium alloys [J]. Trans J Inst Metals, 1977, 18(3): 277-288.
[5] HU X S, ZHANG Y K, ZHENG M Y, WU K. A study of damping capacities in pure Mg and Mg-Ni alloys [J]. Scripta Materialia, 2005, 52(11): 1141-1145.
[6] MAKER G L, KRUGER J. Special issue on platform science and technology for advanced magnesium alloys [J]. Int Mater Rev, 1993, 38(3): 138-153.
[7] LAMBRI O A, RIEHEMANN W, LUCIONI E J, BOLMARO R E. Mechanical spectroscopy of deformed WE43 magnesium alloys [J]. Mater Sci Eng A, 2006, 442: 476-479.
[8] LEE J K, YOON Y O, KIM S K. Development of CaO added wrought Mg alloy for cleaner production [C]//LUO A A, NEELAMEGGHAM N R, BEALS R S. Proceedings of Magnesium Technology. The Minerals, Metals and Materials Society, 2006: 185-189.
[9] KIM S K. Effect of alkaline earth metal oxides in magnesium alloys [C]//LUO A A, NEELAMEGGHAM N R, BEALS R S. Proceedings of Magnesium Technology. The Minerals, Metals and Materials Society, 2006: 517-521.
[10] KIM S K, LEE J K, YOON Y O, JO H H. Development of AZ31 Mg alloy wrought process route without protection gas [J]. Materials Processing Technology, 2007, 187/188: 757-760.
[11] HA S H, LEE J K, JO H H, JUNG S B, KIM S K. The behavior of CaO and Ca in pure Mg [J]. Rare Metals, 2006, 25(s): 150-154.
[12] NASHIF A D, JONES D I G, HENDERSON J P. Vibration damping [M]. New York: John Wiley & Sons Inc., 1985.
[13] GRANATO A, L?CKE K. Application of dislocation theory to internal friction phenomena at high frequencies [J]. Journal of Applied Physics, 1956, 27: 789-803.
[14] FRANK F C, READ W T. Carnegie institute of technology symposium on the plastic deformation of crystalline solids [M]. Washington, DC: Office of Naval Research, 1950: 44-48.
Corresponding author: Dong-In JANG; E-mail: kpu033@kitech.re.kr
(Edited by LI Yan-hong)


