Sintering technology of Ti(C, N) base cermets
ZHOU Shu-zhu(周书助)1,2, TAN Jin-hao(谭锦灏)2, PENG Wei-zhen(彭卫珍)1,
WANG She-quan(王社权)1, LI Ping(李 萍)1
1. Zhuzhou Cemented Carbide Group Corp. Ltd, Zhuzhou 412000, China;
2. School of Metallurgical Engineering, Hunan University of Technology, Zhuzhou 412008, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
The variations of chemical compositions, phases, microstructure evolution and shrinking of cermets compact debinded in H2 or in vacuum and sintered subsequently in vacuum were studied systematically using chemical analysis, back scattering scanning electron microscopy (SEM), and X-ray diffractometry (XRD). The total carbon of cermets debinded in H2 is lower than that debinded in vacuum by 0.4%-0.5%. The contents of carbon and oxygen are decreased sharply when being sintered at 1 100-1 300 ℃. The decomposition reaction of nitrogen is conducted sharply at 1 300 ℃. However, the decomposition of nitrogen is inhibited while the liquid phase appears, and then begins again above 1 500 ℃. The solution reaction of TaC and Mo2C into ring phase starts at 1 200 ℃, and WC into ring phase at 1 300 ℃ is finished. Therefore, the heating rate during sintering of cermets between 900 ℃ and 1 350 ℃ is important.
Key words:
Ti(C, N) cermets; sintering; composition; shrinkage; microstructure;
1 Introduction
Ti(C, N) base cermets have been developing in recent years which are considered as excellent cutting tools and mould materials, with properties between cemented carbide and ceramic material. Because its raw material is cheap and easily gained, Ti(C, N) base cermets are researched widely in many industrialized countries, especially in Japan[1-3]. Ti(C, N) base cermets have more complex compositions and microstructures than common cemented carbides with higher nitrogen and oxygen contents; and more complex reactions may take place in the sintering process which can affect the composition, microstructure and properties of alloy directly[4-6].
The research on the composition variation, microstructure evolution and compact shrinking of cermets in sintering process is very useful for designing of raw composition and sintering schedule, eliminating structural defect in alloy and improving properties.
2 Experimental
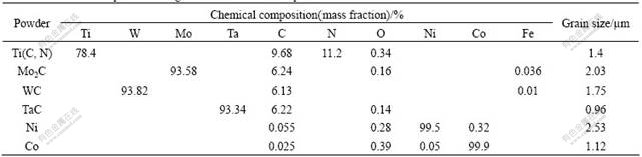
The chemical composition and grain size of the as-received powders are listed in Table 1. The original composition of cermets containing 50%Ti(C, N)-10%WC-10%TaC-10%Mo2C-10%Co-10%Ni (mass fraction) was prepared. The powder mixtures were ball milled in ethyl alcohol for 72 h and blended. The ball-to-material mass ratio was 10:1. The bar specimens using 4%PEG as binder, with 6.2 mm×8.0 mm×25 mm in size were pressed and debinded in H2 or in vacuum under procedure of room temperature-350 ℃, 2 h, 350-380 ℃, 2 h, 380-450 ℃, 1 h and 450 ℃, 0.5 h, then sintered at different temperatures of 600 ℃, 800 ℃, 900 ℃, 1 000 ℃, 1 100 ℃, 1 200 ℃, 1 250 ℃, 1 300 ℃, 1 350 ℃, 1 400 ℃, 1 450 ℃ and 1 520 ℃ in vacuum furnace for 1 h. The length, width and height of the bar specimens sintered were measured, respectively, and the average shrinkage ratio of compacts was calculated (the ratio of the shrinkage of specimens to green bar dimension). The bar specimens sintered at different temperatures were crushed into powders to pass through 175 μm screen mesh for chemical analysis and X-ray diffractometry(the scanning speed is 0.5(?)/s in 20?-75?). The microstructures were observed using FE-SEM.
Table 1 Chemical composition and grain size of as-received powders
3 Results and discussion
3.1 Compositions change of green compacts after debinding
PEG is a common powder-shape binder, and the thermal cracking remnant of pure PEG is 0.03%. When it is heated to 450 ℃ in vacuum, the remnant is very small. There are two debinding atmospheres, vacuum and H2, in this production process of cemented carbide. When PEG was mixed into powder, the remnant of PEG in cermets green compacts is related to its existent state and debinding process.
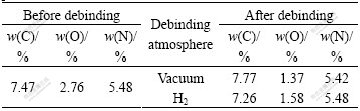
Table 2 lists the compositions of compacts before and after debinding. It can be seen that debinding atmosphere has an important effect on the composition of cermets compact. Compared with the designed composition, the total carbon of cermets compact after debinding in vacuum is increased by 0.2%-0.3%; however, the total carbon of cermets compact after debinding in H2 is decreased by about 0.2%. Therefore, the total carbon of cermets compact debinded in H2 is lower than that in vacuum by 0.4%-0.5%.
The nitrogen content is hardly changed in debinding process. There is about 1.5% combined oxygen in cermets compact. Because there is trace amount of H2O in H2, the oxygen content of cermets compact debinded in vacuum is lower than that debinded in H2.
Table 2 Compositions of compacts before and after debinding process
3.2 Shrinking behavior of cermets sintered in vacuum
The shrinkage of cermets compact sintered at different temperatures is shown in Fig.1. The cermets compact begins to shrink above 600 ℃ due to the atomic diffusion. The shrinkage of compact becomes quick above 900 ℃ due to the solution reaction and quick atomic diffusion. The cermets compact begins to shrink sharply above 1250℃ due to plastic flow, particle rearrangement, dissolving and precipitation of particle after the appearance of liquid phase. The shrinkage of compact approaches the maximum value at about 1 400 ℃, the shrinkage of compact becomes very slow at 1 400-1 450 ℃. When the sintering temperature is above 1 500 ℃, the compact does not shrink but expand, which is contributed to the decomposition of nitrogen at high temperature. Because the decomposition of nitrogen occurs on uncovered Ti(C, N) grain, the pore in alloy becomes larger[7-8].
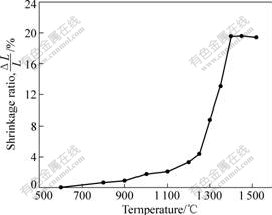
Fig.1 Shrinkage ratio of cermets compact sintered at different temperatures
3.3 Degassing reaction of cermets compact sintered in vacuum
3.3.1 Change of carbon content in compact
The change of chemical composition in cermets compact sintered in vacuum is shown in Fig.2. Debinding process of the green compacts has been finished at 600 ℃, and a little remnant carbon of binding agents may be contained. Because the oxygen of binding phase in green compacts is reduced at 600 ℃, the total carbon content is declined by about 0.1%, compared with designing composition. The total carbon content is declined at 600-1 300 ℃. Firstly, the oxygen in tungsten carbide is reduced at 600-900 ℃, WO3→W4O11 →WO2→W. Then, the oxygen in TaC and Ti(C, N) is reduced at about 1 200 ℃, TiO2→Ti2O3→TiO→Ti.
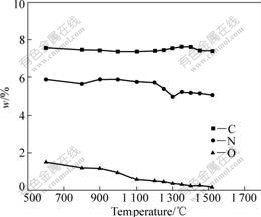
Fig.2 Change of chemical composition of cermets during sintering
When the sintering temperature is above 1 300 ℃, the total carbon content of cermets has little change[9]:
CoO+C→Co+CO↑ (1)
WO2+C→W+CO↑ (2)
Ti(C, N, O)+C→Ti(C, N)+CO↑ (3)
3.3.2 Change of oxygen content in compact
The mainly adsorbing oxygen in green compact is released at 600 ℃. Because of reducing reaction, the oxygen content in green compact declines at 900-1 100 ℃. The oxygen content in compact declines rapidly at 1 100-1 300 ℃, because the reducing reaction is carried out drastically, which reveals there is higher bonding oxygen content in Ti(C, N) powder. The oxygen content in cermets declines gradually when the sintering temperature is above 1 300 ℃. The declining quantity of oxygen content in cermets compact in sintering process is related with loss of total carbon content in it.
3.3.3 Change of nitrogen content in compact
The change of nitrogen content in green compact has little change below 900 ℃. Ti(C, N) reacts with other carbides in solid state to form solid solution and N2 is released above 900 ℃, then the nitrogen content in compact begins to decline. With the increase of sintering temperature, the degassing reaction of N2 is carried out quickly to the peak at about 1 300 ℃. When the liquid phase appears at about 1 350 ℃, the compact densifies rapidly; the opening pores become closed; and the reaction of degassing N2 is inhibited[10]. The effect of binder phase on the decomposing of N2 is that decomposing reaction is accelerated before the liquid phase appears, and the decomposing reaction of N2 is inhibited after the liquid phase appears[1]. When sintering temperature exceeds 1 500 ℃, the decomposing of N2 is carried out again[6, 11].
There is a quick decline of nitrogen content in cermets compact at 1 200-1 300 ℃. The loss of nitrogen content in cermets compacts is about 1%.
3.4 Solid state reaction and phase change of cermets sintered in vacuum
Relative intensities of XRD of the different phases in cermets sintered at different temperatures is shown in Fig.3. The phase compositions in compact have little change below 900 ℃. Mo2C and TaC diffuse and take part in solid state reaction with Ti(C, N) above 900 ℃. With increasing the sintering temperature, the solid state reaction is carried out quickly. The solid state reaction of Mo2C and TaC is finished at 1 200 ℃. The phase of WC has little change below 1 100 ℃, then WC diffuses and takes part in solid state reaction with Ti(C, N) above 1 100 ℃. With increasing the sintering temperature, the amount of WC declines quickly at about 1 300 ℃. When sintering temperature is higher than 1 300 ℃, there are only two phases, Ti(C, N) and Ni(Ni + Co), in the cermets.
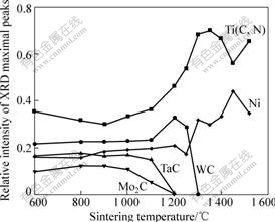
Fig.3 Relative intensities of XRD maximal peaks of different phases in cermets vs sintering temperature
3.5 Microstructure evolution of cermets sintered in vacuum
The microstructure evolution of cermets sintered in vacuum is analyzed by comparing the change of shrink of compact and relative intensities of XRD maximal peak of all phases in compact sintered at different temperatures. When the sintering temperature reaches 1 200 ℃, TaC and Mo2C disappear; the cermets compact begins to shrink; and the atomic diffusion mainly happens in the areas where fine particles are enriched and the particles have a good contact. Particles are distributed in primary state, as shown in Fig.4(a). It can be seen from Fig.4(b) that atomic diffusion and shrink of compact are speeded up; and some Ti(C, N) particles are surrounded by discontinuous or continuous WC as inner ring phase. In Fig.4(c) the pure WC phase begins to disappear. Although the liquid phase does not appear at 1 300 ℃, obvious solid state reaction is
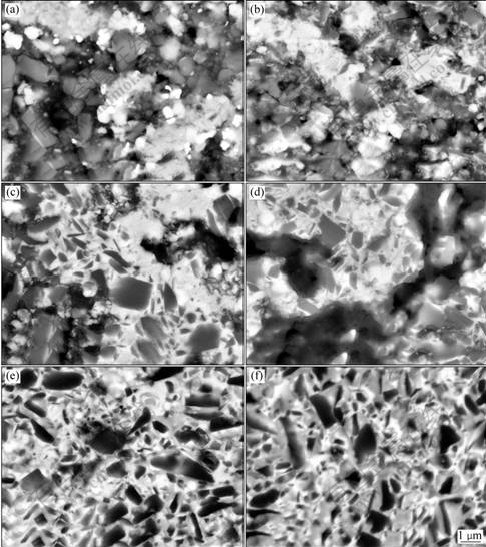
Fig.4 Structure evolution of cermets in sintering process: (a) 1 200 ℃; (b) 1 250 ℃; (c) 1 300 ℃; (d) 1 350 ℃; (e) 1 400 ℃; (f) 1 450 ℃
observed. The inner ring is thickened and alloying begins to appear in part region. At 1 350 ℃, the liquid phase appears; and the atomic diffusion is greatly speeded up. Besides in some bigger pores regions, the cermets compact is alloyed on the whole. At 1 400 ℃, more liquid phases appear; the shrink of cermets speeds up; and cermets nearly densifies.
It can be considered that the following reactions may happen from the relative intensity change of XRD maximal peaks of each phase in cermets compact[6, 12]:
Mo2C+Ti(CXNY)→(Mo, Ti)C+Ti(CUNV) (4)
TaC+Ti(CXNY)→(Ti, Ta)C+Ti(CUNV) (5)
WC+Ti(CXNY)→(Ti, W)C+Ti (CUNV) (6)
Mo2C+WC+TaC+Ti(CXNY)→
(Ti, W, Ta, Mo)C+Ti(CUNV) (7)
(Mo, Ti)C+(Ti, W)C+(Ti, Ta)C+Ti(CXNY)→
(Ti, Mo, W, Ta)(C, N) (8)
Ti(CXNY) and Ti(CUNV) represent different solid solutions with different C-to-N molar ratios. When the sintering temperature exceeds 900 ℃, Eqs.(4) and (5) occur to form (Mo, Ti)C and (Ti, Ta)C. Eqs.(6) and (7) occur above 1 100 ℃, and are finished at about 1 300 ℃.
When the liquid phase appears, the early reaction products, (Mo, Ti)C, (Ti, W)C, (Ti, Ta)C, (Ti, W, Ta, Mo)C, Ti(C, N) and (Mo, Ti)(C,N) diffuse and dissolve in liquid binder phase. The dissolved atoms precipitate on the surface of larger Ti(C, N) particles, forming the ring structure with Ti(C, N) core enriched in N and (Ti, Mo, W, Ta)(C, N) ring, hardly containing N[13]. Above 1 400 ℃, the atomic diffusion and dissolving are more sufficient, and there is not obvious change in whole microstructure.
4 Conclusions
1) The total carbon of cermets compact debinded in H2 is lower than that debinded in vacuum by 0.4%-0.5%, and there is about 1.5% combined oxygen in cermets compact.
2) The total carbon and oxygen contents in cermets compact decline gradually with increasing the sintering temperature. The nitrogen content in compact begins to decline above 1 100 ℃; the degassing peak of N2 is formed at 1 300 ℃; and the decomposition of N2 is accelerated above 1 500 ℃.
3) Mo2C and TaC diffuse and take part in solid state reaction with Ti(C, N) above 900 ℃, and disappear at about 1 200 ℃. WC diffuses and takes part in solid state reaction with Ti(C, N) above 1 100 ℃; and it is dissolved at about 1 300 ℃. There are only two phases, Ti(C, N) and Ni(Ni+Co), in the cermets above 1 300 ℃.
4) There are complicated metallurgy and chemical change in cermets compact sintered at 900-1 350 ℃. In order to gain dense and desired microstructure of Ti(C, N) base cermets, the heating rate must be controlled in this temperature range, and the sintering temperature cannot exceed 1 500 ℃.
Reference
[1] CHEN Li-min, LENGAUER W, ETTMAYER P, DREYER K, DAUB H W, KASSEL D. Fundamentals of liquid phase sintering for modern cermets and functionally graded cemented carbonitrides(FGCC) [J]. International Journal of Refractory Metals and Hard Materials, 2000, 18(6): 307-322.
[2] PARK S, KANG Y J, KWON H J, KANG S. Synthesis of (Ti, M1, M2)(CN)–Ni nanocrystalline powders [J]. International Journal of Refractory Metals and Hard Materials, 2006, 24(1/2): 115-121.
[3] LINDAHL P, GUSTAFSON P, ROLANDER U, STALS L, ANDREN H O. Microstructure of model cermets with high Mo or W content [J]. International Journal of Refractory Metals and Hard Materials, 1999, 17(6): 411-421.
[4] ZHOU Shu-zhu, WANG She-quan, PENG Wei-zheng, WANG Ling-sen. Effect of sintering atmosphere on structure and properties of Ti(CN) base cermets [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(9): 1350-1357.
[5] WON T K, JUNE S P, SHINHOO K. Effect of group IV elements on the cutting characteristics of Ti(C, N) cermet tools and reliability analysis [J]. Journal of Materials Processing Technology, 2005, 166(1): 9-14.
[6] JINKWAN J, SHINHOO K. Effect of ultra-fine powders on the microstructure of Ti(CN)-xWC-Ni cermets [J]. Acta Materialia, 2004, 52(6): 1379-1386.
[7] QI F, KANG S. Study on microstructural changes in Ti(C, N)-NbC-Ni cermets [J]. Mater Sci Eng A, 1998, 251(1/2): 276-285.
[8] FEN Ping, XIONG Wei-hao, YU Li-xin. Phase evolution and microstructure characteristics of ultrafine Ti(C, N)-based cermet by spark plasma sintering [J]. International Journal of Refractory Metals and Hard Materials, 2004, 22(2/3): 133-138.
[9] CHEN Li-min, WALTER L, KLAUS D. Advances in modern nitrogen-containing hardmetals and cermets [J]. International Journal of Refractory Metals and Hard Materials, 2000, 18(2): 153-161.
[10] ETTMAYER P, KOLASKA H, LENGAUER W. Ti(C, N) cermets metallurgy and properties [J]. International Journal of Refractory Metals and Hard Materials, 1995, 13(3): 343-351.
[11] WALLY P, ETTMAYER P, LLENGAUER W. The Ti-Mo-C-N system: Stability of the (Ti, Mo)(C, N)1-X phase [J]. Journal of Alloys and Compounds 1995, 228(1): 96-101.
[12] YANG Jun-kui, CHUL L H. Microstructural evolution during the sintering of a Ti(C, N)-Mo2C-Ni alloy [J]. Mater Sci Eng A, 1996, 209(1/2): 213-217.
(Edited by YANG Bing)
Corresponding author: ZHOU Shu-zhu; Tel: +86-733-2887896; E-mail: zhoushuzhu@126.com



