Trans. Nonferrous Met. Soc. China 22(2012) 2840-2845
Microbial extraction of nickel from chromite overburdens in the presence of surfactant
Sunil Kumar BEHERA, Lala Behari SUKLA
CSIR-Institute of Minerals & Materials Technology, Bhubaneswar 751013, India
Received 11 January 2012; accepted 25 May 2012
Abstract:
The effect of surfactant polyoxyethylenesorbitan monolaurate (Tween-20) on the nickel bioleaching from pre-treated chromite overburden (COB), Sukinda with fungal strain Aspergillus niger, was examined in shake flasks. Along with the nickel recovery from COB by the fungal bioleaching, the effect of surfactant on the growth of the A. niger was also investigated. Results show that the addition of surfactant in low concentration was favorable for the recovery of nickel from pre-treated COB. Normally, the carbon source (sucrose) in the culture medium was utilized by the A. niger for its cellular metabolism and organic metabolites (bio acids) were produced, which were responsible for the bioleaching of minerals. However, the addition of surfactant (Tween-20) accelerated the rate of sucrose consumption by the fungi, and thus enhancing the extraction of nickel from pre-treated COB. During the study, around 39% nickel extraction was achieved in A. niger mediated bioleaching performed at 2% pulp density of pre-treated COB at 30 °C, in the presence of surfactant whereas only 24% nickel was extracted without surfactant.
Key words:
nickel; surfactant; Tween-20; chromite overburden; Aspergillus niger; microbial extraction; bioleaching; cellular metabolism; organic metabolite;
1 Introduction
The nickel bioleaching from lateritic ores has been extensively studied through mineral and microbes interactions [1-4]. Unlike other microorganisms, fungus solubilizes metals from lateritic ore by formation of organic acids viz citric acid, oxalic acid, gluconic acid etc. Filamentous fungi, such as A.niger, major group of brown-rot and white-rot basidiomycete, are able to efficiently produce and secrete large quantities of oxalate [5]. As an alternative to classical methods, laterites can be leached out by organic acids. The organic acids exert the leaching process via acidolysis, complexation and chelate formation [1,6,7]. Therefore, for better recovery of metal values in bio hydrometallurgical operations, selection and development of specific microbial strains are the prime concern.
A. niger produces oxalic acid, citric acid and/or gluconic acid according to the operating conditions [8,9]. Out of several proposed pathways, KUBICEK et al [8] suggested that pyruvate generated during the process of glycolysis is transformed to oxaloacetate. This process is enhanced by supplying relevant quantities of phosphorous and nitrogen sources to the growth medium, operating at pH close to 7 [8]. It has been reported that application of ultrasonic waves for the pre-treatment of A. niger for bioleaching of lateritic nickel resulted in improved nickel extraction [10]. Similarly, the use of ultrasonic waves improved the bio dissolution of metals, such as copper, zinc, cobalt, nickel, iron and aluminium, from black shale with A. niger because of improved organic acid secretion from the fungus under controlled conditions [11]. Micro silicate particles were used as novel tool to control the morphological development of A. niger, for improvement of enzyme and organic acid secretion [12]. Organic acids secreted by A. niger have been proved to be useful in nickel bioleaching [10,13,14].
Much effort has been made to enhance the reaction kinetics of bioleaching. However, the addition of some metal ions cofactors, nutritional supplements like nitrogen and phosphate sources, and surfactants could also improve the bioleaching with fungi. In the present context, it was observed that the surfactant polyoxyethylenesorbitan monolaurate (Tween-20) significantly enhanced the bioleaching of nickel from COB by A. niger.
The Sukinda COB contains 0.8%-1.0% nickel, which is strategically an important metal that needs to be exploited [15]. The extraction of nickel present in goethite matrix of the COB needs to be processed through minimal energy utilization and eco-friendly mineral processing methodology. Since, hydro- metallurgical processes are antagonistic to bio- hydrometallurgy in terms of both in energy utilization and environmental concerns; hence bioleaching has emerged as an alternative to address the challenges in processing of nickel laterites.
In the present work, research was conducted to estimate the nickel recovery with the supplementation of the surfactant polyoxyethylenesorbitan monolaurate to the bioleaching culture medium of A. niger. The comparative data were presented to assess the influence of the surfactant on the fungal bioleaching of nickel from COB.
2 Materials and methods
2.1 Chemical and mineralogical analyses of COB
The COB samples were collected from Sukinda Mines, Odisha, India. The samples were crushed and sieved to obtain particle size less than 75 μm. The samples were air dried and the metal concentrations were determined on an atomic absorption spectrophotometer (AAS) after digesting in concentrated HCl. Mineralogical analyses of the original and leached samples were carried out by means of a diffractometry (Phillips, PW3710) with a radiation operating at 40 kV and 30 mA to identify major and minor minerals. Experiments were performed using pre-treated COB. The pre-treatment of COB was done in a muffle furnace operated at 600 °C for 5 h under normal atmospheric conditions.
2.2 Micro organism and culture conditions
Pure culture of A. niger (MTCC No: 6999) was used for the study. The fungal strain was isolated from COB of Sukinda mines and grown in Bromofield medium (0.75 g/L MgSO4·7H2O, 0.25 g/L KH2PO4, 0.25 g/L (NH4)2SO4, 1 g/L yeast extract and 20 g/L sucrose), with initial pH 6.8 at 30 °C. Spore suspension around 106 spore/mL of 5-7 d-old culture was used as inoculum for further experimentation.
Culture filtrates were collected during bioleaching process and were analyzed by HPLC (Model-Agilent- 1100, Agilent technologies, Waldbronn Analytical Division, Germany) to quantify the amount of organic acid produced. Zorbax Eclipse XDB-C18 column (150 mm × 4.6 mm, i.d) was used. The organic acids were quantified by UV detection at 210 nm with diode array detector (DAD, Model -G 1315A) (17).
2.3 Oxaloacetate degradation assay
The assay for oxaloacetate hydrolysis activity was performed as reported by LENZ et al [16]. The reaction solution (2 mL) contained 0.125-1.0 mmol/L oxaloacetate, 0.5 mmol/L MnCl2, crude cytoplasmic extract of A. niger containing oxaloacetate acetylhydrolase (OAH), and 0.1 mol/L imidazole (pH 7.6 and temperature 25 °C). The reaction was monitored at 255 nm for the disappearance of enol tautomer of oxaloacetate. Prior to estimation of the reaction rate catalyzed by the enzyme containing cell extract of the A. niger, the rate of oxaloacetate consumption via spontaneous decarboxylation was measured to determine the background rate. The actual reaction rate was measured by subtracting the background rate from the reaction rate measured in the presence of enzyme.
2.4 Bioleaching experiments
Leaching experiments were carried out in triplicate with 250 mL Erlenmeyer flasks containing 90 mL of Bromofield medium supplemented with surfactant polyoxyethylenesorbitan monolaurate (Tween-20) with concentration ranging from 0 to 0.05 mL/L. Each flask was inoculated with 10 mL (106 spore/mL) of spore suspension with 2% (w/v) pre-treated COB and incubated for 24 d at 30 °C on a rotary shaker at 150 r/min. Initial pH of the medium was 6.8. Samples were drawn at regular intervals and were analyzed by AAS to determine the percentage of nickel leached in the solution. Control experiments were also performed without addition of fungal suspension and surfactant. Aseptic conditions were maintained throughout the experiment.
3 Results and discussion
3.1 Characterization of COB
The detail chemical analysis of COB shows that the raw COB contains 0.99% nickel whereas after pre-treatment of COB, the nickel content is 1.02%. The composition map of COB sample shows that nickel occurred in an absorbed state within the goethite (iron oxide) matrix. Pre-treatment of COB results in complete conversion of goethite into hematite phase, subsequently leading to homogeneous distribution of nickel particles throughout the matrix [17]. The surface area of the ore particle increased due to pre-treatment of COB, the initial surface area was (23.2±0.5) m2/g and after pre-treatment the surface area of COB particle changed to (45.1±0.5) m2/g. X-ray diffraction pattern of the pre-treated COB indicated that the peaks associated with goethite which was originally present in the raw ore had almost disappeared and there was also a marked increase in intensity of peak band of hematite. As a result of development of micro pores and cracks in the ore particles, better percolation of lixiviate or microbial metabolites into the matrix of mineral for metal bioleaching was observed.
3.2 Leaching study
Figure 1 represents the plot for nickel extraction from pre-treated COB using A. niger with different concentrations of surfactant as a function of time. All the experiments were conducted at 2% (w/v) pulp density with Bromofield medium containing 20 g/L sucrose. About 39% of nickel was recovered from COB by A. niger in the presence of 0.05 mL/L surfactant, whereas the nickel recovery was only 24% without surfactant in A. niger culture. 34% nickel was recovered from thermally treated COB by A. niger bioleaching conducted with culture medium containing 100 g/L sucrose, so our investigation revealed that the application of surfactant reduced the requirement of sucrose, i.e, the carbohydrate source for leaching purpose [18]. The result shows that the nickel recovery was increased with application of surfactant to fungal culture medium, keeping other parameters constant.
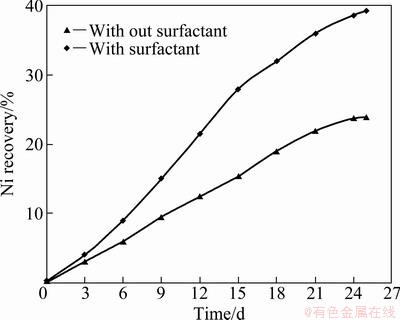
Fig. 1 Nickel recovery from pre-treated COB by A. niger in the presence of surfactant (0.05 mL/L) and without Tween-20 in shake flask scale
Cellular metabolism of A. niger converts carbon sources in the medium into variety of products including organic acids which lead to lowering of pH. Organic acids, such as oxalic acid, citric acid, gluconic acid, are excreted into the medium as metabolic products by the heterotrophs and subsequently dissolve heavy metals by forming salts and chelates. HPLC results indicated the presence of both oxalic along with a trace amount of citric acid found in the culture filtrates of A. niger. During the study, it was observed that the oxalic acid concentration (0.423 g/L) was more in the culture filtrate of A. niger grown in the presence of surfactant than that (0.291 g/L) in leaching medium without surfactant. Elaborate study was carried out for the production of oxalic acids since oxalic acid is one of the most potential organic acids in leaching of the lateritic COB minerals [2].
The growth study of A. niger shows that about (35.1±0.5) g of wet biomass was generated in 100 mL culture containing 0.05 mL/L surfactant after 12 d whereas without the application of surfactant, (34.8±0.5) g of wet mass per 100 mL A. niger culture medium was produced. A marginal difference in biomass generation was observed with reference to the supplement of surfactant in the medium. Higher nickel recovery in the presence of surfactant showed enhanced sucrose consumption rate in culture medium. Figure 2 reflects that up to the 3rd day of bioleaching by A. niger, the rate of sucrose consumption was the same in both the cases but beyond that the rate of sucrose utilization increased in the presence of the surfactant. The average size of fungal micelle after 72 h of growth in surfactant was 2-3 mm, whereas the average size of fungal micelle was 5-6 mm without surfactant (Fig. 3). The smaller size micell of A. niger provided more surface area for mineral-microbe interaction. Hence, the microbial metabolic lixiviates generated at mineral-microbe interface interacted more efficiently upon mineral matrix, as a result of which it improved the nickel extraction from pre-treated COB.
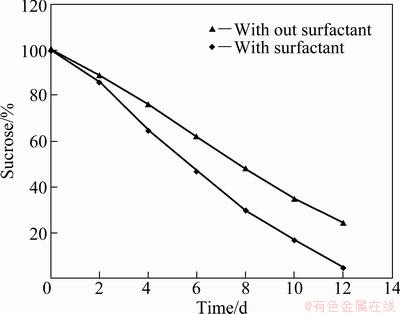
Fig. 2 Sucrose concentration in culture medium of A. niger during bioleaching
It was anticipated that addition of surfactant to culture medium may affect the enzymatic activity to produce more oxalate. Oxalate can be formed from oxaloacetate in a C—C lysis reaction catalyzed by oxaloacetate hydrolase (oxaloacetate acetylhydrolase, OAH, EC 3.7.1.1). The key enzyme oxaloacetate acetylhydrolase is located in the cytoplasm of A. niger, which catalyzes conversion of oxaloacetate to oxalate and acetate [8]. The crude cell extract of A. niger cultured both in the presence and absence of surfactant showed no significant difference in oxaloacetate hydrolysis activity (Fig. 4).
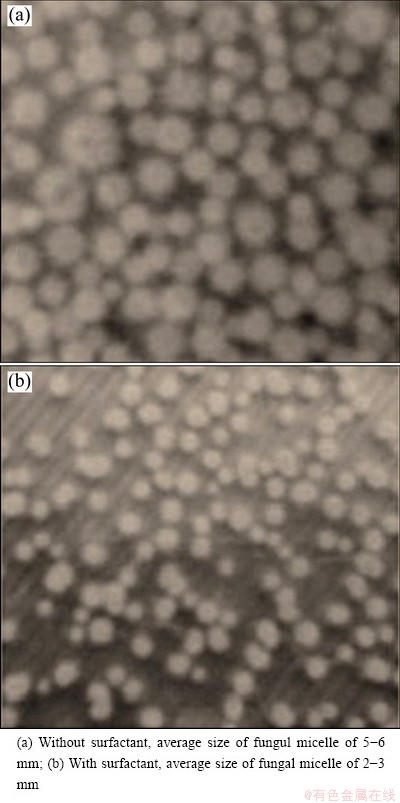
Fig. 3 Micell of fungus A. niger after 72 h of incubation
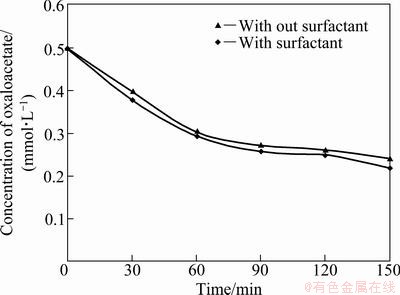
Fig. 4 Rate of oxalate synthesis from oxaloacetate metabolic intermediate by catalytic activity of crude cell extract from A. niger grown both in the presence and absence of Surfactant
3.3 Reaction kinetics
Nickel is associated with the goethite iron matrix of the COB [17]. In the present study, nickel was leached out from the COB matrix due to the attack of fungal metabolites or the bio acids (oxalic and citric acid) generated by the fungi during cellular metabolism. In the present context, the kinetics of the A. niger mediated bioleaching process has been studied to support the reaction mechanism. Various kinetic models, such as chemical, diffusion and mixed control, were applied to studying the kinetics of nickel bioleaching. When the rate of metal extraction is controlled by the chemical dissolution of the ore particles by bio-acids produced by the fungus, then the following rate Eq. (1) is generally applied, by assuming the shape of the ore particle as spherical [19].
1-(1-α)1/3=k1t (1)
However, if the nickel diffusion is very fast, the kinetics of leaching usually obeys three-dimensional diffusion equation of the following type.
1-(2/3)α-(1-α)2/3=k2t (2)
where α is the leaching fraction of nickel at time t; k1 and k2 are the reaction constants in chemical controlled and three-dimensional diffusion model, respectively.
Nickel bioleaching data obtained in the presence of surfactant revealed that there was a substantial rise in nickel recovery after the 9th day of leaching due to the availability of higher concentration of fungal bio-acids secreted by A. niger. Also the role of the smaller size fungal micell generated in the presence of surfactant may not be neglected. In the presence of the surfactant when the size of micell of A. niger was comparatively smaller, the surface area of microbe-mineral interaction increased, which further favored the higher nickel extraction. Therefore, it may be interpreted from the kinetics study for the process in the presence of surfactant, the initial rate i.e. up to the 9th day (Fig. 5(a)) of the reaction is controlled by chemical dissolution model, beyond that (9th day) the reaction rate was more precisely controlled by the three-dimensional diffusion model (Fig. 5(b)). Whereas for the overall rate kinetics for nickel extraction without surfactant followed chemical control model (Fig. 6). The shifting of rate kinetics from chemical control to three-dimensional diffusion model after the 9th day in surfactant assisted bioleaching might be due to cumulative effect of secreted fungal bio-acids along with higher surface area of microbe-mineral interaction, which favors the higher nickel extraction. In the case of the bioleaching process without surfactant, the overall reaction rate is controlled by chemical dissolution model, which depends upon the lixiviates i.e. the fungal bio-acids which are responsible for the dissolution of metals.
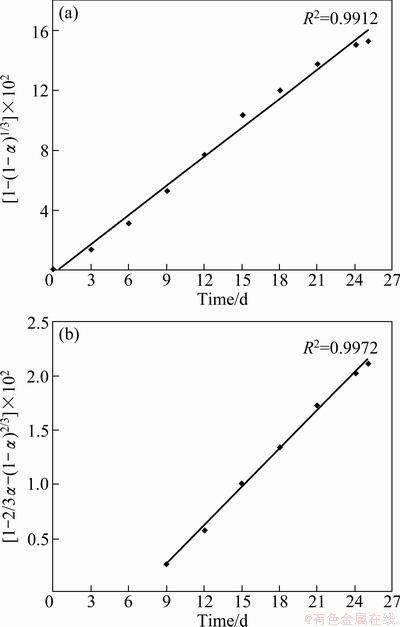
Fig. 5 Application of Eq. (1) (a) and Eq. (2) (b) for nickel extraction rate by A. niger in the presence of surfactant
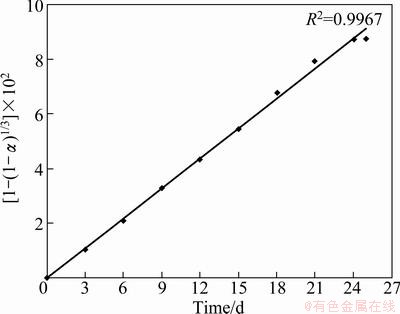
Fig. 6 Application of Eq. (1) for nickel rate by A. niger without surfactant
4 Conclusions
The surfactant polyoxyethylenesorbitan mono- laurate (0.05 mL/L) supplemented to the culture medium of A. niger promotes fungal growth through improvement of sucrose consumption rate by the fungi and also produces comparative smaller size of fungal micell which provides higher surface area for reaction between microbe and mineral. The presence of surfactant also increases the bio-acid secretion by the fungi through the elevated carbohydrate transformation cumulatively effect on higher nickel recovery from pre-treated COB, namely about 39% nickel was extracted by A. niger in the presence of the surfactant where as only 24% nickel extraction was achieved without surfactant.
Acknowledgements
The authors would like to thank Prof. B. K. MISHRA (Director, IMMT) for giving his constant support and encouragement throughout the work. The first author would like to acknowledge CSIR for providing Senior Research Fellowship.
References
[1] BOSECKER K. Bioleaching: metal solubilization by microorganisms [J]. Fems Microbiology Reviews, 1997, 20: 591-604.
[2] SUKLA L B, PANCHANADIKAR V V, KAR R N. Microbial leaching of lateritic nickel ore [J]. World Journal of Microbiology and Biotechnology, 1993, 9: 255-257.
[3] TZEFERIS P G. Leaching of a low-grade hematitic laterite ore using fungi and biologically produced acid metabolites [J]. International Journal of Mineral Processing, 1994, 42: 267-283.
[4] VALIX M, USAI F, MALIK R. Fungal bioleaching of low grade laterite ore [J]. Minerals Engineering, 2001, 14 (2): 197-203.
[5] DUTTON, EVANS C S. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment [J]. Can J Microbiol, 1996, 42: 881-895.
[6] BURGSTALLER W, SCHINNER F. Leaching of metals with fungi [J]. Journal of Biotechnology, 1993, 17: 91-116.
[7] TSEKOVA K, TODOROVA D, GANEVA S. Removal of heavy metals from industrial wastewater by free and immobilized cells of Aspergillus niger [J]. International Biodeterioration & Biodegradation, 2010, 64: 447-451.
[8] KUBICEK C P, KUNAR G S,  M. Evidence for a cytoplasmatic pathway of oxalate biosynthesis in Aspergillus niger [J]. Appl Environ Microbiol, 1988, 54: 633-637.
M. Evidence for a cytoplasmatic pathway of oxalate biosynthesis in Aspergillus niger [J]. Appl Environ Microbiol, 1988, 54: 633-637.
[9] KUBICEK C P, ROHR M. Citric acid fermentation [J]. CRC Crit Rev Biotechnol, 1986, 3: 331-373.
[10] SUKLA L B, SWAMY K M, NARAYANA K L, KAR R N,PANCHANADIKAR V V. Bioleaching of Sukinda laterites using ultrasonics [J]. Hydrometallurgy, 1995, 37(3): 387-391.
[11] ANJUM F, BHATTI H N, GHAUR M A. Enhanced bioleaching of metals from black shale using ultrasonics [J]. Hydrometallurgy, 2010, 100: 122-128.
[12] HABIB D, BECKY S, CHRISTOPH W. Morphology engineering of aspergillus niger for improved enzyme production [J]. Biotechnology and Bioengineering, 2010, 105(6): 1058-1068.
[13] ANJUM F, BHATTI H N, ASGHER M, SHAHID M. Leaching of metal ions from black shale by organic acids produced by Aspergillus niger [J]. Applied Clay Science, 2010, 47: 356-361.
[14] MEHTA K D, DAS C, PANDEY B D. Leaching of copper, nickel and cobalt from Indian Ocean manganese nodules by Aspergillus niger [J]. Hydrometallurgy, 2010, 105: 89-95.
[15] SUKLA L B, DAS R P. Kinetics of nickel dissolution from pre treated laterites [J]. Trans Indian Inst Met, 1987, 40: 351-353.
[16] LENZ H, WUNDERWALD P, EGGERER H. Partial purification and some properties of oxalacetase from Aspergillus niger [J]. Eur J Biochem, 1976, 65: 225-236.
[17] BEHERA S K, PANDA P P, SINGH S, PRADHAN N, SUKLA L B, MISHRA B K. Study on reaction mechanism of bioleaching of nickel and cobalt from lateritic chromite overburdens [J]. International Biodeterioration and Biodegradation, 2011, 65: 1035-1042.
[18] MOHAPATRA S, BOHIDAR S, PRADHAN N, KAR R N, SUKLA L B. Microbial extraction of nickel from Sukinda chromite overburden by Acidithiobacillus ferridans and Aspergillus strains [J]. Hydrometallurgy, 2007, 85: 1-8.
[19] HABASI F. Principles of extractive metallurgy, first principles, vol. 1 [M]. New York: Gordon and Breach, 1969: 413-420.
在表面活性剂存在下铬铁矿表土中镍的微生物萃取
Sunil Kumar BEHERA, Lala Behari SUKLA
CSIR-Institute of Minerals & Materials Technology, Bhubaneswar 751013, India
摘 要:加入表面活性剂吐温20,用Aspergillus niger对预处理过的印度Sukinda铬铁矿表土在摇瓶中生物浸取镍。考察添加表面活性剂吐温20对黑曲霉菌Aspergillus niger生长及浸镍效果的影响。结果表明,添加低浓度的表面活性剂吐温20对黑曲霉菌从预处理过的铬铁矿表土中提取镍是有利的。通常,Aspergillus niger利用培养基中的碳源来进行细胞代谢,产生有机代谢物,从而生物浸出矿。添加表面活性剂吐温20加速了黑曲霉菌对碳源的消耗, 从而改善了镍浸出效果。在预处理矿浆浓度为2%和温度为30 °C的条件下,添加表面活性剂吐温20的镍浸取率能达到39%,没有表面活性剂的镍浸取率只有24%。
关键词:镍;表面活性剂;Tween-20;铬铁矿表土;黑曲霉菌Aspergillus niger;微生物萃取;生物浸取;细胞代谢;有机代谢物
(Edited by YANG Hua)
Corresponding author: Sunil Kumar BEHERA; Tel: +91-674-2581635, +91-674-2581638 (Ext.528); E-mail: skbehera2020@gmail.com
DOI: 10.1016/S1003-6326(11)61540-9
Abstract: The effect of surfactant polyoxyethylenesorbitan monolaurate (Tween-20) on the nickel bioleaching from pre-treated chromite overburden (COB), Sukinda with fungal strain Aspergillus niger, was examined in shake flasks. Along with the nickel recovery from COB by the fungal bioleaching, the effect of surfactant on the growth of the A. niger was also investigated. Results show that the addition of surfactant in low concentration was favorable for the recovery of nickel from pre-treated COB. Normally, the carbon source (sucrose) in the culture medium was utilized by the A. niger for its cellular metabolism and organic metabolites (bio acids) were produced, which were responsible for the bioleaching of minerals. However, the addition of surfactant (Tween-20) accelerated the rate of sucrose consumption by the fungi, and thus enhancing the extraction of nickel from pre-treated COB. During the study, around 39% nickel extraction was achieved in A. niger mediated bioleaching performed at 2% pulp density of pre-treated COB at 30 °C, in the presence of surfactant whereas only 24% nickel was extracted without surfactant.


