Trans. Nonferrous Met. Soc. China 24(2014) 3818-3824
Electric field assisted chemical conversion process of AZ91D magnesium alloy in HCO3-/CO32- solution
Zhi-xin BA1,2, Xiao-bo ZHANG1,2, Zhang-zhong WANG1,2, Xin-xian FANG1,2, Qiang-sheng DONG1,2, Qiang WANG3
1. College of Materials Engineering, Nanjing Institute of Technology, Nanjing 211167, China;
2. Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology, Nanjing 211167, China;
3. Jiangsu Konsung Equipment Co., Ltd., Danyang 212300, China
Received 17 October 2013; accepted 4 November 2014
Abstract:
A new method for synthesizing Mg-Al hydrotalcite conversion coating on AZ91D Mg alloy was developed by the application of electric field (EF). By using EF technique, the formation time of the coating can be significantly reduced. The SEM results indicate that a continuous and compact Mg-Al hydrotalcite coating is formed on the surface of Mg alloy after short time EF treatment. However, a long time treatment would make the coating partially exfoliate. The corrosion current density (Jcorr) of the coated sample (EF1+1 h) is approximately two orders of magnitude lower than that of Mg alloy substrate. The test of electrochemical impedance spectroscopy (EIS) and immersion corrosion also suggest that the coating can effectively protect Mg alloy against corrosion.
Key words:
magnesium alloy; Mg-Al hydrotalcite; conversion coating; corrosion resistance; electric field;
1 Introduction
Magnesium is an attractive material for manufacture of light parts due to its low density and excellent physical and mechanical properties [1]. These characteristics make Mg-based alloys extremely attractive in vehicle applications [2]. However, the poor corrosion resistance of Mg alloys limits their applications [3,4]. Surface treatment is a basic method to improve the corrosion resistance of magnesium alloys. Typically, the conversion coatings (CCs) are most widely used because they are easy to perform and cost-effective [5]. The chromate conversion coating provides excellent corrosion protection, but hexavalent chromium is a toxic substance that pollutes environment and is detrimental to health [6]. Several promising chromate-free CCs have been investigated, including phosphate [7], phosphate- permanganate [8], stannate [9], rare earth salt [10,11], calcite [12] and others. However, the above studies did not take account of the environmental problems associated with heavy metal ions and phosphorus. Moreover, impurities from the above mentioned CCs may contaminate the magnesium melt [13-15]. LIN et al [16] explored a carbonic acid conversion process which fabricated a Mg-Al hydrotalcite film on AZ91D by immersing the sample in aqueous HCO3-/CO32- solution in a simple, economical and environmentally friendly process, but it took at least 12 h. In their recent studies [17-19], the treatment time was reduced to 4 h by immersing AZ91D Mg alloy in acidic and alkaline HCO3-/CO32- bath for 2 h successively. For comparison, conventional conversion coating treatments of Mg alloy require only 0.5-60 min [20]. Therefore, an efficient and clean approach should be developed.
In recent works, the improvement effects of EF on the formation of CC on AZ91D Mg alloy have been reported [21-24]. ELSENTRIEY et al [21,22] have reported the formation of uniform stannate CC with high corrosion resistant on AZ91D Mg alloy by using the potentiostatic technique. They found that anodic polarization during the stannate conversion process accelerated dissolution of magnesium ions and promoted deposition of the coating. An alternative voltage treatment technique has been used to fabricate stannate CC on AZ91D Mg alloy by LIU et al [23]. The results showed that a continuous and compact dual-layer CC with excellent corrosion resistance was formed. WANG et al [24] presented that introduction of a cathodic component during the micro-arc oxidation process was helpful to obtain a uniform and compact coating. However, little is known about the effect of the EF on the formation and microstructure of the carbonic acid conversion coating on Mg alloys.
In the present work, a constant potential is applied to the carbonic acid conversion coating formation process. It is thought that EF will accelerate dissolution of Mg anode, which provides the source of Mg2+ and Al3+ for Mg-Al hydrotalcite. Meanwhile, the hydrogen evolution reaction near cathode becomes quick, which will shorten the time of carbonic acid solution reaching an alkaline condition. The alkaline solution will promote the Mg-Al hydrotalcite film formation [18]. Additionally, the directional movement of OH- and CO32- to Mg anode under EF in carbonic acid will also promote Mg-Al hydrotalcite deposition. The effect of EF on the microstructure and the corrosion resistance of the carbonic acid conversion coating was studied. Compared with the immersion conversion process, the accelerated effect of EF assisted process was also investigated.
2 Experimental
2.1 Preparation of Mg-Al hydrotalcite conversion coated sample
The specimens used for the investigation were die- cast AZ91D alloy sheet with dimensions of 20 mm×25 mm×5 mm. The working surface was wetly ground using SiC paper of 700, 1000 and 1500 grid, degreased with ethyl alcohol in an ultrasonic cleaner for 10 min, rinsed with distilled water in an ultrasonic cleaner for 5 min, and dried in a compressed hot air flow. The carbonated water was prepared at room temperature by bubbling CO2 (99.99%) gas through 1000 mL of deionized water. The flow rate of CO2 gas was 2 L/min. It took 10 min to minimize the pH of the solution (pH ~4.0). The specimens immersed in the acidic HCO3-/CO32- solution at 50 °C under EF treated from 1 to 5 h were denoted as EF1h, EF2h, EF3h, EF4h and EF5h. In another experiment, the samples were immersed in carbonic acid solution at 50 °C while CO2 gas was continually bubbled through the solution first, and then were dipped in the HCO3-/CO32- solution with a high pH 11.5 which was produced by dropwise addition of 1.25 mol/L aqueous NaOH with vigorous stirring during the mixing. Assisted by EF, the AZ91D sample underwent carbonic acid solution treatment for 1 h first, and then was dipped into HCO3-/CO32-solution at pH 11.5 at 50 °C for 1 h. This treatment was denoted as EF1+1 h. For comparison, immersion (IM) conversion coating sample was carried out in acidic and alkaline HCO3-/CO32- bath for 2 h successively, which was denoted by IM2+2 h. Each sample was treated in the bath with 100 mL of HCO3-/CO32- solution.
For EF assisted treatment, the AZ91D samples and the nickel plate (99.9%) acted as the anode and cathode respectively. The samples were embedded in resin and the exposed surface was about 200 mm2. The distance between the samples and cathode was maintained at about 25 mm. A cell tester was employed to maintain a constant voltage (0.2 V).
2.2 Characterization
The crystallographic structure of the specimens was analyzed by X-ray diffraction (XRD) (UltimaIV, Rigaku) with Cu Kα (1.5405  ) radiation. The microstructure of the samples was studied by means of JSM-6360LV scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectrum (EDS).
) radiation. The microstructure of the samples was studied by means of JSM-6360LV scanning electron microscope (SEM) equipped with an energy dispersive X-ray spectrum (EDS).
Electrochemical polarization tests, electrochemical impedance spectroscopy (EIS) and immersion tests were employed to determine the corrosion resistance of samples. All electrochemical measurements were made using an Ametek Parstat 2273 Potentiostat/Galvanostat/ FRA and powersuit software. For electrochemical measurements, a three-electrode cell was used with a saturated calomel electrode (SCE) as a reference electrode and platinum flake as counter electrode. The area of the coating exposed to the solution was 1 cm2. Electrochemical polarization tests were performed in a corrosion cell that contained 300 mL of 3.5% NaCl solution at a scan rate of 0.5 mV/s. EIS results for the samples were also measured in 3.5% NaCl solution in a frequency range from 100 kHz to 10 mHz and the perturbing AC amplitude was 5 mV. All the experiments were repeated more than three times to show the better reproducibility. The immersion tests were performed according to the GB 10124—88. All the tests were performed at room temperature.
3 Results and discussion
3.1 XRD analysis and morphology of Mg-Al hydrotalcite coating
Figure 1 shows the pH of the aqueous HCO3-/CO32- solution during EF treatment in this work. As shown in Fig. 1, the pH value increases rapidly from 4.0 to 6.0 in 1 h treatment. Subsequently, the pH value slowly increases from 6.0 to 8.1 in 4 h treatment. After treatment for 4 h, the pH value slightly increases from 8.1 to 8.5 in 1 h. Comparatively, it takes at least 12 h to increase the pH value from 4.3 to 8.5 during IM treatment in the research of LIN and UAN [17]. This indicates that the EF treatment will shorten the time of carbonic acid solution reaching an alkaline condition. In a sense, EF treatment will shorten the formation time for a crystalline Mg-Al hydrotalcite coating on AZ91D alloy.
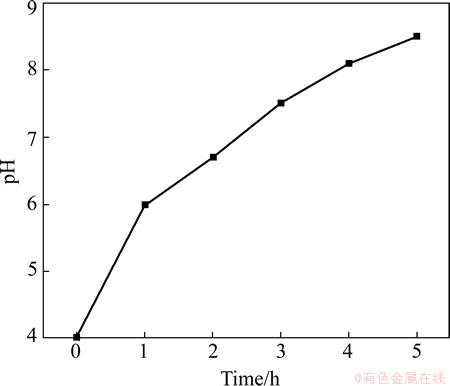
Fig. 1 pH value of aqueous HCO3-/CO32- solution during EF treatment
The XRD patterns of the samples treated by EF method for different time and by IM method are presented in Fig. 2. As shown in Fig. 2, X-ray peaks of Mg-Al hydrotalcite (Mg6Al2(OH)16CO3·4H2O) (JCPDS No.41-1428) are observed after being treated by EF and IM methods. When the treatment time in acidic HCO3-/CO32- bath increases from 1 to 4 h, the X-ray peaks of Mg-Al hydrotalcite become stronger gradually. But as the treatment time is prolonged to 5 h, the X-ray peaks of Mg-Al hydrotalcite become weak obviously. The acidic/alkaline successive treatment process causes the XRD pattern to yield peaks of Mg-Al hydrotalcite, and peaks of EF1+1 h sample are similar to those of IM2+2 h sample.

Fig. 2 XRD patterns of samples treated by EF method for different time and by IM method
Figure 3 displays SEM images of IM2+2 h sample and the samples treated by EF method for different time. Figure 3(a) reveals an incomplete coating with some fine cracks after 1 h treatment. Increasing the treatment time to 4 h, a double-layer coating forms on the sample. As described in Fig. 3(b), the bottom layer is a continuous coating with finer arc-shaped cracks than EF1h sample and the upper layer is a discontinuous coating. However, when the treatment time is prolonged to 5 h, the surface crack size increases and the coating is locally chipped (white box in Fig. 3(c)). The chemical composition of the white box region in Fig. 3(c) is determined by EDS. It reveals that the region is composed of Mg and Al, which means that the Mg substrate is exposed after the coating chips. During EF assisted treatment, the formation of a Mg-Al hydrotalcite structure on the AZ91D sample in HCO3-/CO32- solution is strongly related to the pH value, similar to the previous reports of UAN et al [16-19]. With the treatment time prolonging from 1 to 4 h, the pH of aqueous HCO3-/CO32- increases from 4.0 to 8.1 (see Fig. 1), the X-ray peaks of Mg-Al hydrotalcite become stronger (see Fig. 2) and the coating becomes more uniform and compact (see Figs. 3(a) and (b)). Notably, the HCO3-/CO32- solution after 4 h has become alkaline (see Fig. 1(a)). However, formation of the crystalline Mg-Al hydrotalcite coating needs at least 12 h during IM treatment [16-19]. Therefore, the accelerated effect of EF on the formation of crystalline Mg-Al hydrotalcite coating is significant. As the treatment time increases to 5 h, the pH continuously raises to 8.5, but weaker X-ray peaks of Mg-Al hydrotalcite from the sample than 4 h are obtained (see Fig. 2) due to partially exfoliation of the coating (see Fig. 3(c)). Under EF assisted treatment, the anode dissolves quickly and provides more Mg2+ and Al3+ which can prompt the formation of Mg-Al hydrotalcite coating. Nevertheless, when a continuous and compact coating is formed on the AZ91D after 4 h, the migration of Mg2+, Al3+, OH- and CO32- is impeded, and then the formation speed of the coating decreases. When the dissolution speed of the AZ91D exceeds the formation speed of Mg-Al hydrotalcite, Mg2+ and Al3+ concentrate between AZ91D substrate and the coating. At that time, coating breaks off locally. As mentioned above, EF assisted treatment accelerates the formation of crystalline Mg-Al hydrotalcite coating in HCO3-/ CO32-solution, but long time treatment deteriorates the quality of coating.
The surface morphologies of EF1+1 h and IM2+2 h samples which are all treated successively in acidic/ alkaline HCO3-/CO32- solution are shown in Figs. 3(d) and (e). It can be seen that the two samples have more compact coatings, but still exhibit several network-like cracks. The inserted figures in Figs. 3(d) and (e) all show typical sheet-like morphology of Mg-Al hydrotalcite. However, the lamellar in EF1+1 h surface is finer and denser than that of IM2+2 h sample. Figure 3 (f) shows the cross-sectional microstructure of EF1+1 h sample. The thickness of the Mg-Al hydrotalcite coating is 4–6 μm. It also can be seen that some fine cracks are observed in the coating, but the cracks do not penetrate the layer directly to the substrate metal, which can avoid the exposure of substrate metal to the environment. YU et al [18] claimed that strong alkaline can prompt the transformation of precursor layer into a crystallized Mg-Al hydrotalcite. In EF assisted process, the strong alkaline will further shorten the formation time of crystalline Mg-Al hydrotalcite which can avoid the coating deterioration for long time treatment. As a result, a similar compact and uniform coating can be gained by EF or IM method, but the treatment time of EF method is just half of IM method.
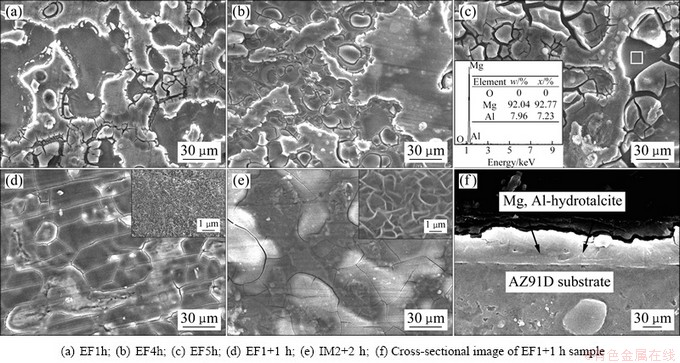
Fig. 3 Surface morphologies of samples treated by different methods
3.2 Corrosion behavior of Mg-Al hydrotalcite coating
The polarization curves of the die-cast AZ91D sample, IM2+2 h sample and EF samples treated for different time measured in 3.5% NaCl solution are plotted in Fig. 4. The corrosion potential (φcorr) of the EF4h sample is -1.45 V, while that of the AZ91D substrate is -1.51 V. Based on the polarization measurements, the corrosion current density Jcorr of the AZ91D substrate is about 2×10-4 A/cm2. The Jcorr decreases firstly and then increases with prolonging the treatment time in acidic HCO3-/CO32- solution. The lowest value is 1.41×10-5 A/cm2 for EF4h, which is decreased by about one order of magnitude than that of the AZ91D substrate. The Jcorr of the EF5h is 5.85×10-5 A/cm2 which is higher than that of EF4h. As shown
in Fig. 4, when acidic/alkaline treatment is used successively, the φcorr of EF1+1 h is increased to -1.43 V and that of the IM2+2 h sample is -1.41 V. The Jcorr of EF1+1 h is down to about 7×10-6 A/cm2 and that of the IM2+2 h is about 5×10-6A/cm2.
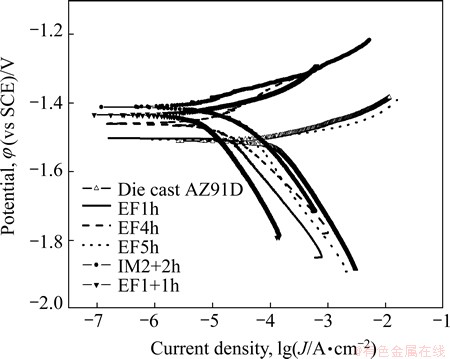
Fig. 4 Polarization curves of samples treated by EF method for different time, IM2+2h sample and die-cast AZ91D sample in 3.5% NaCl solution
Figure 5(a) shows typical EIS for die-cast AZ91D sample, IM2+2 h sample and EF samples treated for different time. As displayed in the inset in Fig. 5(a), the plot for the AZ91D substrate comprises one high frequency capacitive loop and one low frequency inductive loop. The total capacitive loop is related to the polarization resistance of the coating in NaCl aqueous solution, while the high frequency capacitive loop is due to the contribution of electric double layer at the interface of substrate and solution, and the low frequency inductive loop is attributed to the corrosion nucleation in the initiation stage of localized corrosion [13]. The existence of inductive loop indicates that there are corrosion pits on the surface of Mg substrate. The curves for the coated samples change with the treatment time and the treatment method. The low frequency inductive loop which exists in the curves of EF1h and EF5h is not obvious in the curve of EF4h. The curves for the EF1+ 1 h and IM2+2 h samples contain only one capacitive loop. This implies that the coatings are compact and undamaged. A higher polarization resistance typically corresponds to a lower corrosion rate. The polarization resistance of the AZ91D substrate is ~0.4 kΩ·cm2. As presented in Fig. 5(a), the EF1h sample has a resistance of about 1.7 kΩ·cm2, which is much lower than that of the EF4h sample (~8 kΩ·cm2). The polarization resistance of EF1+1 h sample is ~10.5 kΩ·cm2, which is about thirty times that of the AZ91D substrate. Though the polarization resistance of EF1+1 h sample is slightly poorer than that of IM2+2 h sample (~11.3 kΩ·cm2), the treatment time of EF1+1 h sample is just half that of IM2+2 h sample.
The equivalent circuits of EIS are applied to simulating the impedance curves of the AZ91D substrate and EF1+1 h sample, as shown in Figs. 5 (b) and (c). Re represents the solution resistance; R1 is the charge transfer resistance; Q1 represents the electric double layer capacity and Q2 is the capacity of the Mg-Al hydrotalcite film on the AZ91D alloy. The constant phase element (Q) is used to compensate for the non-homogeneity in the system [25]. Q is defined by two values, Y0 and n. If n is equal to 1, Q is identical to a capacitor. The inductive behavior of the AZ91D substrate is specified by the resistance R2 and inductance L, which are used to describe the low frequency inductive loop, implying the initiation of pitting corrosion [25]. The capacity and resistance of the Mg-Al hydrotalcite coating on the AZ91D alloy are respectively characterized by Q2 and R3.
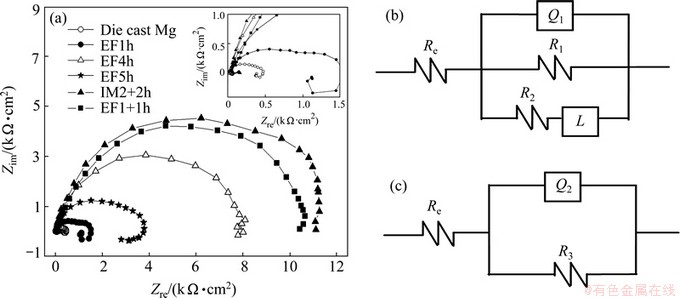
Fig. 5 EIS results of samples treated by EF method for different time, IM2+2 h sample and die-cast AZ91D sample measured in 3.5% NaCl solution (a), equivalent circuit of die-cast AZ91D sample (b) and equivalent circuit of EF1+1 h sample (c)

Fig. 6 Optical corrosion morphologies of EF1+1 h sample (a) and die-cast AZ91D sample (b) after immersion in 3.5% NaCl solution for 72 h
Figure 6 displays the optical corrosion morphologies of the EF1+1 h sample and die-cast AZ91D sample after 72 h immersion test. The surface of EF1+1 h sample shows small corrosion spots after the immersion test. For comparison, as shown in Fig. 6 (b), the AZ91D substrate suffers seriously localized corrosion. Therefore, the Mg-Al hydrotalcite coating formed by EF assisted technique effectively increases the corrosion resistance of the AZ91D Mg alloy in NaCl environment. Moreover, the immersion test (see Fig. 6) results are qualitatively consistent with the electrochemical polarization scanning (see Fig. 4) and the EIS measurement (see Fig. 5).
4 Conclusions
1) The formation time of a crystalline Mg-Al hydrotalcite conversion coating can be significantly reduced by the application of EF technique. The EF can accelerate dissolution of Mg anode and evolution of hydrogen near the cathode, which provides the source of Mg2+, Al3+ and OH- for Mg-Al hydrotalcite. Meanwhile, the directional movement of OH- and CO32- to Mg anode under EF also promotes coating formation.
2) The EF has strong influence on the microstructure of conversion coating. A continuous and compact coating is formed on AZ91D alloy surface after short time EF treatment, but a long time treatment makes the coating partially exfoliate.
3) The Mg-Al hydrotalcite coating formed by EF assisted technique can effectively protect the Mg substrate against corrosion. The corrosion current density of the Mg-Al hydrotalcite coated sample is about 7×10-6 A/cm2, which is roughly two orders of magnitude less than that of the Mg substrate (2×10-4 A/cm2). The EIS measurement and immersion corrosion test are consistent with the electrochemical polarization scanning.
References
[1] THIRUGNANASELVI S, KUTTIRANI S, EMELDA A R. Effect of schiff base as corrosion inhibitor on AZ31 magnesium alloy in hydrochloric acid solution [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1969-1977.
[2] BEN HAMU G, ELIEZER D, WAGNER L. The relation between severe plastic deformation microstructure and corrosion behavior of AZ31 magnesium alloy [J]. Journal of Alloys and Compounds, 2009, 468(1-2): 222-229.
[3] AGNEW S R, NIE J F. Preface to the viewpoint set on: The current state of magnesium alloy science and technology [J]. Scripta Materialia, 2010, 63(7): 671-673.
[4] WU G, IBRAHIM J M, CHU P K. Surface design of biodegradable magnesium alloys—A review [J]. Surface & Coatings Technology, 2013, 233: 2-12.
[5] CHEN Xiao-bo, BIRBILIS N, ABBOT T B. Review of corrosion-resistant conversion coatings for magnesium and its alloys [J]. Corrosion, 2011, 67(3): 1-16.
[6] GREY J E, LUAN B, Protective coatings on magnesium and its alloys—A critical review [J]. Journal of Alloys and Compound, 2002, 336 (1-2): 88-113.
[7] CUI Xue-jun, LIU Chun-hai, YANG Rui-song, LI Ming-tian, LIN Xiu-zhou, GONG Min. Phosphate film free of chromate, fluoride and nitrite on AZ31 magnesium alloy and its corrosion resistance [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(11): 2713-2718.
[8] ZUCCHI F, FRIGNANI A, GRASSI V, TRABANELLI G, MONTICELLI C. Stannate and permanganate conversion coatings on AZ31 magnesium alloy [J]. Corrosion Science, 2007, 49(12): 4542-4552.
[9] HAMDY A S, BUTT D P. Novel smart stannate based coatings of self-healing functionality for AZ91D magnesium alloy [J]. Electrochimica Acta, 2013, 97(1): 296-303.
[10] LEE Y L, CHEN Y R, CHEN F J, LIN C S. Mechanism of the formation of stannate and cerium conversion coatings on AZ91D magnesium alloys [J]. Applied Surface Science, 2013, 276(7): 578-585.
[11] CHEN Dong-chu, WU Jian-feng, LIANG Yi-qing, YE Shu-lin, LI Wen-fang. Preparation of cerium oxide based environment-friendly chemical conversion coating on magnesium alloy with additives [J]. Transactions of Nonferrous Metals of China, 2011, 21(8): 1905-1910.
[12] YU B L, PAN X L, UAN J Y. Enhancement of corrosion resistance of Mg-9wt.% Al-1wt.% Zn alloy by a calcite (CaCO3) conversion hard coating [J]. Corrosion Science, 2010, 52(5): 1874-1878
[13] CHEN Jun, SONG Ying-wei, SHAN Da-yong, HAN En-hou. Properties of dawsonite conversion film on AZ31 magnesium alloy [J]. Transactions of Nonferrous Metals of China, 2011, 21(4): 936-942.
[14] CHEN Jun, SONG Ying-wei, SHAN Da-yong, HAN En-hou. Study of the in situ growth mechanism of Mg,Al-hydrotalcite conversion film on AZ31 magnesium alloy [J]. Corrosion Science, 2012, 63(12): 148-158.
[15] LI Wei-ping, WANG Xi-mei, ZHU Li-qun, LI Wen. Formation mechanism of an aluminum based chemical conversion coating on AZ91D magnesium alloy [J]. International Journal of Minerals, Metallurgy and Materials, 2010, 17(5): 641-647.
[16] LIN J K, HSIA C L, UAN J Y. Characterization of Mg,Al-hydrotalcite conversion film on Mg alloy and Cl- and CO32- anion-exchangeability of the film in a corrosion environment [J]. Scripta Materialia, 2007, 56(11): 927-930.
[17] LIN J K, UAN J Y. Formation of Mg,Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3-/CO32- and corresponding protection against corrosion by the coating [J]. Corrosion Science, 2009, 51(5): 1181-1187.
[18] YU B L, LIN J K, UAN J Y. Recent studies on applications of carbonic acid solution for developing conversion coating on Mg alloy [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(7): 1331-1339.
[19] LIN J K, JENG K L, UAN J Y. Crystallization of a chemical conversion layer that forms on AZ91D magnesium alloy in carbonic acid [J]. Corrosion Science, 2011, 53(11): 3832-3839.
[20] HUO Hong-wei, LI Ying, WANG Fu-hui. Corrosion of AZ91D magnesium alloy with a chemical conversion coating and electroless nickel layer [J]. Corrosion Science, 2004, 46(6): 1467-1477.
[21] ELSENTRIEY H H, AZUMI K, KONNO H. Improvement in stannate chemical conversion coatings on AZ91D magnesium alloy using the potentiostatic technique [J]. Electrochimica Acta, 2007, 53(2): 1006-1012.
[22] ELSENTRIEY H H, AZUMI K, KONNO H. Effects of pH and temperature on the deposition properties of stannate chemical conversion coatings formed by the potentiostatic technique on AZ91D magnesium alloy [J]. Electrochimica Acta, 2008, 53(12): 4267-4275.
[23] LIU Xiao-lan, ZHANG Tao, SHAO Ya-wei, MENG Guo-zhe, WANG Fu-hui. Effect of alternating voltage treatment on the microstructure and corrosion resistance of stannate conversion coating on AZ91D alloy [J]. Corrosion Science, 2009, 51(11): 2685-2693.
[24] WANG Shu-yan, XIA Yong-ping, LIU Li, SI Nai-chao. Preparation and performance of MAO coatings obtained on AZ91D Mg alloy under unipolar and bipolar modes in a novel dual electrolyte [J]. Ceramics International, 2014, 40(1): 93-99.
[25] CAO Chu-nan, ZHANG Jian-qing. Introduction of electrochemical impedance spectroscopy [M]. Beijing: Science Press, 2002. (in Chinese).
电场辅助AZ91D镁合金碳酸水溶液化学转化工艺
巴志新1, 2,章晓波1, 2,王章忠1, 2,方信贤1, 2,董强胜1, 2,王 强3
1. 南京工程学院 材料工程学院,南京 211167;
2. 江苏省先进结构材料与应用技术重点实验室,南京 211167;
3. 江苏康尚医疗器械有限公司,丹阳 212300
摘 要:开发一种在AZ91D镁合金表面制备镁合金镁铝水滑石膜的电场辅助新工艺。该工艺可有效缩短镁铝水滑石转化膜的成膜时间。扫描电镜结果表明:短时间的电场辅助处理可在镁合金表面形成一层连续、致密的镁铝水滑石膜,但长时间处理会使膜层出现局部脱落现象。电场辅助处理2 h试样(EF1+1h)的腐蚀电流密度比镁合金基体低约2个数量级,交流阻抗测试和浸泡腐蚀试验结果均表明,该膜层能有效地保护镁合金。
关键词:镁合金;镁铝水滑石;转化膜;耐蚀性;电场
(Edited by Yun-bin HE)
Foundation item: Project (12KJB430007) supported by the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province, China; Projects (CKJB201203, CKJA201202) supported by the Innovation Fund of Nanjing Institute of Technology China; Project (201311276001Z) supported by the Innovative Foundation Project for Student of Nanjing Institute of Technology, China
Corresponding author: Xiao-bo ZHANG; Tel: +86-15951722675; E-mail: xbxbzhang2003@163.com
DOI: 10.1016/S1003-6326(14)63538-X
Abstract: A new method for synthesizing Mg-Al hydrotalcite conversion coating on AZ91D Mg alloy was developed by the application of electric field (EF). By using EF technique, the formation time of the coating can be significantly reduced. The SEM results indicate that a continuous and compact Mg-Al hydrotalcite coating is formed on the surface of Mg alloy after short time EF treatment. However, a long time treatment would make the coating partially exfoliate. The corrosion current density (Jcorr) of the coated sample (EF1+1 h) is approximately two orders of magnitude lower than that of Mg alloy substrate. The test of electrochemical impedance spectroscopy (EIS) and immersion corrosion also suggest that the coating can effectively protect Mg alloy against corrosion.


