Trans. Nonferrous Met. Soc. China 24(2014) 257-262
Recovery of indium by acid leaching waste ITO target based on neural network
Rui-di LI, Tie-chui YUAN, Wen-bo FAN, Zi-li QIU, Wen-jun SU, Nan-qian ZHONG
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 4 June 2013; accepted 23 October 2013
Abstract:
The optimized leaching techniques were studied by technical experiment and neural network optimization for improving indium leaching rate. Firstly, effect of single technical parameter on leaching rate was investigated experimentally with other parameters fixed as constants. The results show that increasing residual acidity can improve leaching rate of indium. Increasing the oxidant content can obviously increase leaching rate but the further addition of oxidant could not improve the leaching rate. The enhancement of temperature can improve the leaching rate while the further enhancement of temperature decreases it. Extension leaching time can improve the leaching rate. On this basis, a BPNN model was established to study the effects of multi-parameters on leaching rate. The results show that the relative error is extremely small, thus the BPNN model has a high prediction precision. At last, optimized technical parameters which can yield high leaching rate of 99.5% were obtained by experimental and BPNN studies: residual acidity 50-60 g/L, oxidant addition content 10%, leaching temperature 70 °C and leaching time 2 h.
Key words:
indium; leaching rate; ITO waste target; BPNN model;
1 Introduction
Indium-tin oxide (ITO) thin film is a semiconductor optoelectronic material, possessing special physical properties of visible light transmission, electric conduction, high hardness and chemical stability. Therefore, the ITO film has been used in various optoelectronic equipments for transparent and conductive layer, such as liquid crystal display (LCD), plasma display panel (PDP), organic light emitting diode (OLED) and touch panel. All over the world, almost 60% indium production was used for the preparation of ITO films [1]. Usually, the ITO film was prepared by a DC magnetron sputtering technology using ITO targets. Nevertheless, 85% of the ITO target, which needs to be recovered, could not be utilized for magnetron sputtering process [2]. Moreover, the Sn element also existed in ITO target, because the ITO target is a compound of In2O3 and SnO2 with the mass ratio of 9:1. The other elements are less in the ITO film. It is also essential to separate tin from indium firstly, with the purpose of recovery of indium from waste ITO target [3].
Previous literatures have reported the recovery of indium from ITO target. TOMII and TSUCHIDA [4] described an effective leaching method for indium recovery. KRAJEWSKI and HANUSH [5] demonstrated a technology for solvent extraction of indium from acidic or alkaline hydrous solutions. HSIEH et al [6] developed an efficient and simple method for recovery of indium by hydrometallurgical and hot immersion. ZHANG et al [7] recovered indium using TiO2 particles to absorb indium ions from solution. LIU et al [8] recovered indium using 2-ethylhexyl phosphoric acid mono ester. Overall, among the above methods, the leaching and precipitation method has a high recovery rate and high technical stability, thus it is suitable for industrial production. Therefore, studying the leaching principle and improving leaching rate are necessary.
The usual leaching process uses hydrochloric acid to leach indium oxide. During the process, leaching rate was influenced by many factors which are very complicated and have coupling effect. The complex relationship between leaching rate and technical parameters, therefore, is non-linear and difficult to find its mathematical model, while the artificial neural network could be used to approach a non-linear system. The artificial neural network has the abilities of associative learning, parallel processing and fault tolerance. In recent years, back propagation neural network (BPNN) has been widely used for processing optimization [9,10]. Based on this, BPNN was adopted to optimize the leaching techniques.
In the present work, the effects of various technical parameters on indium leaching rate were experimentally studied. Based on experimental results, the prediction model of leaching rate was developed with BPNN. Then the optimal technical parameters for obtaining a maximal leaching rate were obtained and were experimentally verified.
2 Experimental
Figure 1 shows the flow chart of indium recovery of waste ITO target. The ITO target was firstly smashed and then leached using oxidant and hydrochloric acid. Figure 2 shows the flow chart of indium recovery from tin residue which was obtained from acid leaching in Fig. 1. The relative density of waste ITO target was 98% and the chemical composition is listed in Table 1, which was measured by ICP-AES. The waste ITO target was processed by caustic washing, drying, and smashing, respectively. This experiment was conducted by heating water bath and the temperature was controlled by electronic thermostat instrument with the error of ±1 °C. The experimental process used motor agitation and vacuum pump filtration for liquid-solid separation. The material drying adopted electric heating oven with the temperature error of ±1 °C.
3 Results and discussion
The purpose of this experiment is to leach indium as much as possible. However, the residual content of HCl, the added oxidant amount, leaching temperature, leaching time and other factors all have marked influence on the leaching rate of indium. So, the following work is to study the influence of various factors on the leaching rates of indium, in order to leach the maximal amount of indium.
3.1 Effect of residual acidity on leaching rate
The residual acidity means the residual acid concentration in solution after chemical reaction of leaching. In this experiment, the added quantity of waste ITO target, added amount of oxidant, leaching temperature, leaching time were fixed as constants, while the added HCl content was varied, in order to investigate the effect of residual acidity on leaching rate. Figure 3 shows the effect of residual acidity on leaching rate. Improving residual acidity can enhance the leaching rate of indium. When the residual acidity increases to 50 g/L, the leaching effect tends to be gentle and the increasing rate of leaching rate is not obvious.
Simultaneously, with the further increase of HCl content, the corrosion of equipment aggravated, and the environment pollution was more serious. Thus, considering all factors, the residual acidity of 50-60 g/L can yield the high leaching rate.
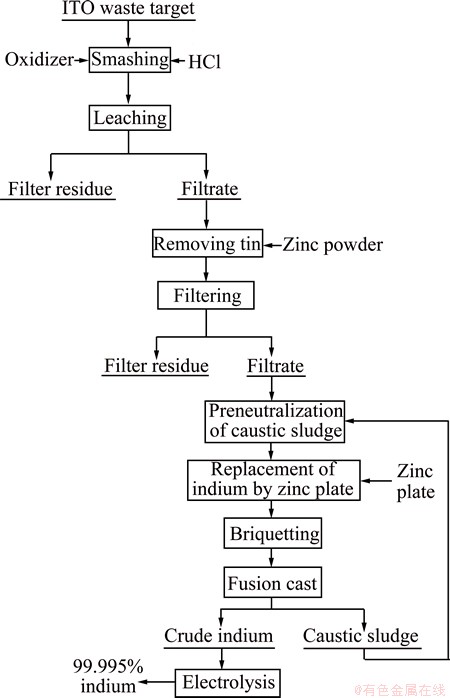
Fig. 1 Flow chart of recovery of indium from waste ITO target
3.2 Effect of added oxidant content on leaching rate
The amount of added ITO powder, residual acidity, reaction temperature and reaction time were fixed as constants, while the added oxidant content, which was proportional to amount of waste ITO powder, was varied. The effect of added oxidant content on leaching rate was studied, as shown in Fig. 4. Obviously, it can be seen that the addition of oxidant can pronouncedly increase leaching rate of indium from ITO waste target. When the oxidant content reached 10%, high leaching rate of 99.7% was obtained. The further addition of oxidant could not obviously improve the leaching rate. Therefore, the added oxidant content of 10% can lead to a high leaching rate exceeding 97.0%.
3.3 Effect of temperature on leaching rate
Figure 5 shows the effect of leaching temperature on leaching rate. It is obvious that the leaching temperature has noticeably influence on leaching rate. In the temperature range of 50-70 °C, the enhancement of temperature can significantly improve the leaching rate from 87% to 99%. However, at the temperature of 70-80 °C, the further increase of temperature cannot increase the leaching rate.
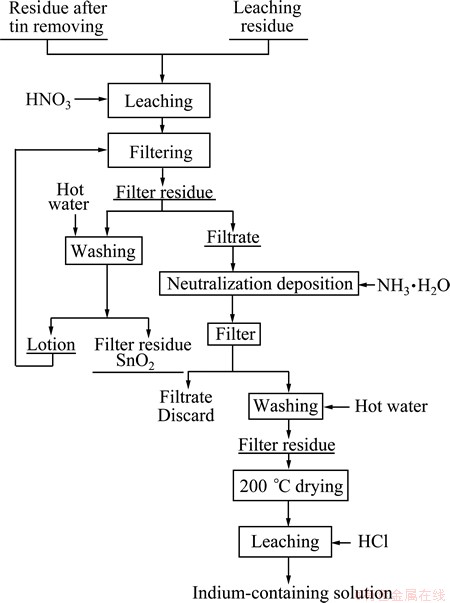
Fig. 2 Flow chart of indium recovery from tin residual obtained by indium recovery from waste ITO target
Table 1 Chemical composition of waste ITO target (mass fraction, %)
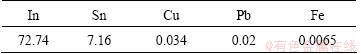
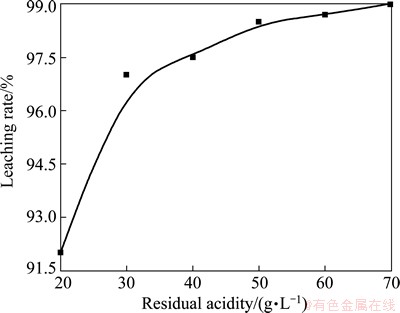
Fig. 3 Effect of residual acidity on leaching rate with other parameters fixed as constants
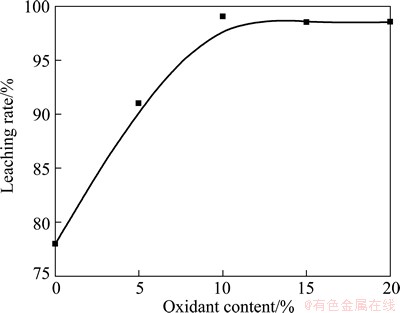
Fig. 4 Effect of oxidant content on leaching rate with other parameters fixed as constants
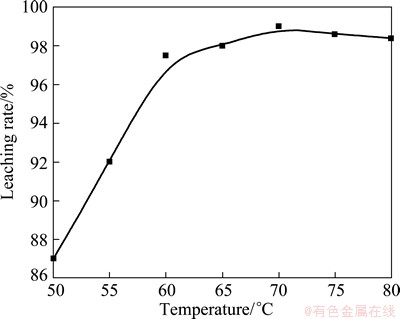
Fig. 5 Effect of temperature on leaching rate with other parameters fixed as constants
3.4 Effect of reaction time on leaching rate
The effect of reaction time on leaching rate was studied, as shown in Fig. 6. It can be seen that the extension time improves the leaching rate. After 2 h, the reaction is basically completed thus the further extension reaction time cannot improve the leaching rate. Therefore, the optimal leaching time should be selected bellow 3 h, which can yield 99.5% of leaching rate.
4 BPNN optimization for leaching technical parameters
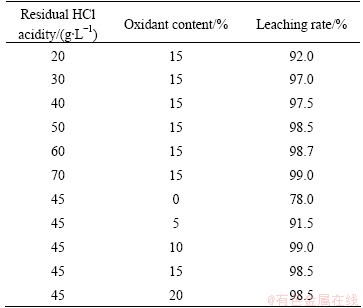
In the present work, the artificial neural network (ANN) with back-propagation (BP) learning algorithm was established to optimize the leaching technical parameters [11]. The residual acidity and oxidant content belong to leaching liquid composition and have very close relationship. Moreover, leaching temperature and time belong to chemical reaction kinetic parameters. Therefore, two BPNN models were established for optimizing leaching liquid composition and reaction kinetic parameters, respectively. In the first BPNN, residual acidity and added oxidant content were used as input variables, while the leaching rate was taken as the output. In another BPNN, leaching temperature and leaching time were used as input variables with leaching rate as output. Table 2 and Table 3 show the input and output variables.
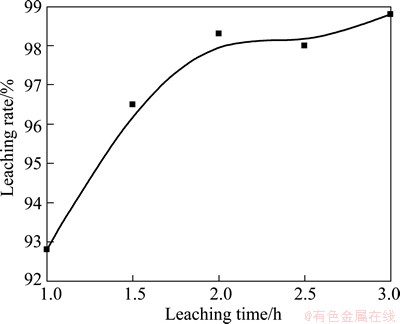
Fig. 6 Effect of leaching time on leaching rate with other parameters fixed as constants
Table 2 Effect of acidity and oxidant content on leaching rate
In order to speed up the convergence of the training network, the original data were transformed into the range of 0 to 1 by the following equation:
 (1)
(1)
where X is the primary technical parameter; Xnorm, Xmax and Xmin are normalized, maximal and minimal values of X, respectively.
Table 3 Effect of leaching time and temperature on leaching rate

In the output layer of BP model, a linear function was used as the transfer function. The transfer functions of hidden layers adopted tan-sigmoid and log-sigmoid functions, which are as follows:
f(x)=tansig x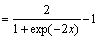 (2)
(2)
f(x)=lgsig x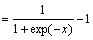 (3)
(3)
The mean square error (MSE) was used to minimize the average squared error between the real output and desired values. The BPNN parameters were selected by trail-and-error, because there is no theoretical method to determine the values. Traditionally, the learning rate and the momentum constants are set to be 0 to 1 [12-14]. It is essential to select an appropriate learning rate. When the learning rate is too high, the calculation is quick but it may reach an unstable state. However, when the learning rate is too low, the calculation will need long time. Thus, it is important to select an appropriate learning rate which can guarantee the stability and training time simultaneously. In the present work, the learning rate of 0.1 was selected.
By BPNN prediction, the effects of technical parameters on leaching rates were obtained. Figure 7 shows the effects of leaching liquid composition and leaching kinetic parameters on leaching rates. Figure 8 shows the contour maps of three-dimensional graph which also shows the effects of leaching liquid composition and leaching kinetic parameters on leaching rate. The optimal parameters can be revealed from which it can yield high leaching rate. It can be seen that the optimal leaching liquid composition are residual acidity 50-60 g/L and oxidant content 10%-20%. The optimal leaching kinetic parameters are temperature 65-80 °C and 2.2-3 h. The above optimal technical parameters can yield high leaching rates exceeding 98.6%.
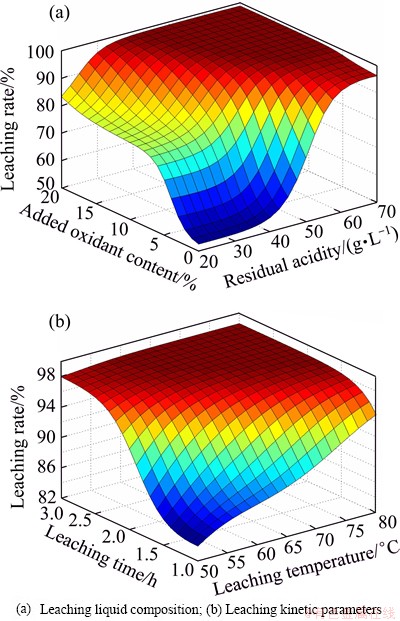
Fig. 7 Effects of technical parameters on leaching rates
5 Verification for BPNN optimization
By the BPNN optimization, the optimal leaching technical parameters were selected as follows: residual acidity 50-60 g/L, oxidant content 10%, leaching time 2.2 h and leaching temperature 70 °C. In order to verify the optimal parameters, the leaching experiment was conducted using above optimal parameters. Two ITO materials of waste ITO powder and waste ITO target were selected. The composition of waste ITO powder is as follows: 74.95% In, 6.96% Sn, and trace elements of Pb, Cu and Fe. The composition of waste ITO target is as follows: 75.02% In, 5.77% Sn, and trace element of Pb, Cu and Fe. Before the leaching experiment, the ITO target was crashed. The detailed experimental conditions are listed in Tables 4 and 5.
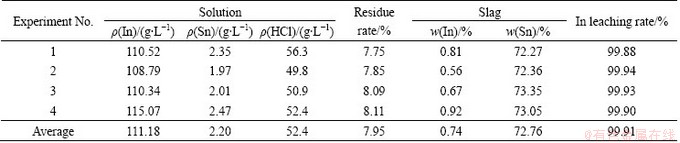
It can be seen that no matter ITO powder or ITO target, the optimal technical parameters can yield high leaching rates exceeding 99.5%. While the BPNN predicts the leaching rate of 98.6%. It can be seen the error is 1.0%-1.3%, which shows good agreement between the experimental and BPNN predicted results.
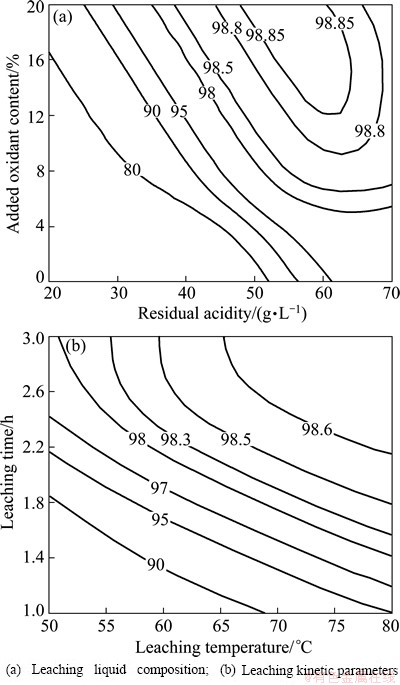
Fig. 8 Contour maps showing effects of technical parameters on leaching rates
6 Conclusions
1) The effects of various single technical parameters on leaching rate were investigated with other parameters fixed as constants. Improving residual acidity can enhance the leaching rate of indium. Increasing the oxidant content can pronouncedly enhance the indium leaching rate but the further addition of oxidant cannot improve it. In the temperature range of 50-70 °C, the enhancement can significantly improve the leaching rate from 87% to 99%, but the further increase of temperature cannot increase the leaching rate. Extension leaching time can improve the leaching rate.
Table 4 Leaching condition of ITO powder
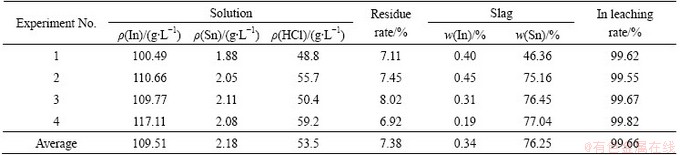
Table 5 Leaching condition of ITO target
2) A BPNN model was established to predict the optimal indium leaching rate. The results show that the average relative error between the predicted and experimental leaching rates is small. Therefore, the BPNN model can be used as an accurate model for predicting of leaching rate.
3) By the experimental and BPNN studies, the optimal leaching rate was obtained as follows: residual acidity of 50-60 g/L, oxidant addition content of 10%, leaching temperature of 70 °C, leaching time of 2 h. Using above technical parameters, a high leaching rate of 99.5% can be obtained.
References
[1] ALFANTAZI A M, MOSKALYK R R. Processing of indium: A review [J]. Minerals Engineering, 2003, 16(8): 687-694.
[2] CHEN J, YAO J S, ZHOU Y Y, CHEN Z F, WANG X, HUANG J W. Recovery indium from waste ITO target [J]. Chinese Journal of Rare Metals, 2003, 27(1): 101-103.
[3] LI Y H, LIU Z H, LI Q H, LIU Z Y, ZENG L. Recovery of indium from used indium-tin oxide (ITO) targets [J]. Hydrometallurgy, 2011, 105(3-4): 207-212.
[4] TOMII K, TSUCHIDA H. Solvent extraction recovery process for indium: US, 4292284[P]. 1981.
[5] KRAJEWSKI W, HANUSH K. Process for fluid-fluid extraction of gallium, germanium or indium from liquid solution: US, 4666686[P]. 1987.
[6] HSIEH S J, CHEN C C, SAY W C. Process for recovery of indium from ITO scraps and metallurgic microstructures [J]. Materials Science and Engineering A, 2009, 158(1-3): 82-87.
[7] ZHANG L, WANG Y N, GUO X J, YUAN Z, ZHAO Z Y. Separation and preconcentration of trace indium(III) from environmental samples with nanometer-size titanium dioxide [J]. Hydrometallurgy, 2009, 95(1-2): 92-95.
[8] LIU J S, CHEN H, CHEN X Y, GUO Z L, HU Y C, LIU C P, SUN Y Z. Extraction and separation of In(III), Ga(III) and Zn(II) from sulfate solution using extraction resin [J]. Hydrometallurgy, 2006, 82(3-4): 137-143.
[9] HUANG Y, BLACKWELL P L. Prediction of mechanical properties of superplastic Inconel* 718 using artificial neural networks [J]. Materials Science and Technology, 2002, 18(10): 1104-1108.
[10] LIAO T W, CHEN L J. Manufacturing process modeling and optimization based on multi-layer perceptron network [J]. Journal of Manufacturing Science Engineering, 1998, 120(1): 109-119.
[11] YANG Xia-wei, ZHU Jing-chuan, NONG Zhi-sheng, HE Dong, LAI Zhong-hong, LIU Ying, LIU Fa-wei. Prediction of mechanical properties of A357 alloy using artificial neural network [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(3): 788-795.
[12] ALMEIDA L M, LUDERMI T B. A multi-objective memetic and hybrid methodology for optimizing the parameters and performance of artificial neural networks [J]. Neurocomputing, 2010, 73(7-9): 1438-1450.
[13] AHMAD J S, TWOMEY J. ANN constitutive model for high strain-rate deformation of Al 7075-T6 [J]. Journal of Materials Processing Technology, 2007, 186(1-3): 339-345.
[14] KANTI K M, RAO P S. Prediction of bead geometry in pulsed GMA welding using back propagation neural network [J]. Journal of Materials Processing Technology, 2008, 200(1-3): 300-305.
神经网络优化ITO废靶酸浸回收铟
李瑞迪,袁铁锤,范文博,邱子力,苏文俊,钟楠骞
中南大学 粉末冶金国家重点实验室,长沙 410083
摘 要:以提高铟浸出率为目标,通过工艺实验结合神经网络研究ITO废靶酸浸的优化工艺。首先,固定其他工艺因素,进行单因素如残酸度、氧化剂加入量、酸浸温度及时间对铟浸出率影响的实验研究。结果表明,增大残酸度可提高铟浸出率;氧化剂的加入可明显提高铟浸出率,但增加到一定程度后浸出率提高不明显;升高温度可明显提高浸出率,但继续升高则会降低铟浸出率;延长浸出时间也可提高铟浸出率。通过反向传播算法的人工神经网络(BPNN)研究多因素的综合作用对铟浸出率的影响规律,预测值与实验值相差很小,表明所建立的BP模型铟浸出率能比较准确地预测。最终,通过BPNN预测以及实验验证,获得高达99.5%浸出率的工艺参数:残酸度50~60 g/L、氧化剂含量10%、浸出温度70 °C和浸出时间2 h。
关键词:铟;浸出率;ITO废靶;人工神经网络模型
(Edited by Xiang-qun LI)
Foundation item: Project (2012BAE06B01) supported by the National Key Technologies R&D Program of China
Corresponding author: Tie-chui YUAN; Tel: +86-731-88830142; E-mail: tiechuiyuan@csu.edu.cn
DOI: 10.1016/S1003-6326(14)63055-7
Abstract: The optimized leaching techniques were studied by technical experiment and neural network optimization for improving indium leaching rate. Firstly, effect of single technical parameter on leaching rate was investigated experimentally with other parameters fixed as constants. The results show that increasing residual acidity can improve leaching rate of indium. Increasing the oxidant content can obviously increase leaching rate but the further addition of oxidant could not improve the leaching rate. The enhancement of temperature can improve the leaching rate while the further enhancement of temperature decreases it. Extension leaching time can improve the leaching rate. On this basis, a BPNN model was established to study the effects of multi-parameters on leaching rate. The results show that the relative error is extremely small, thus the BPNN model has a high prediction precision. At last, optimized technical parameters which can yield high leaching rate of 99.5% were obtained by experimental and BPNN studies: residual acidity 50-60 g/L, oxidant addition content 10%, leaching temperature 70 °C and leaching time 2 h.


