
Preparation of long alumina fibers by sol-gel method using malic acid
TAN Hong-bin, GUO Cong-sheng
School of Materials Science and Engineering, Shaanxi University of Technology, Hanzhong 723003, China
Received 19 July 2010; accepted 15 December 2010
Abstract:
The precursor sol of alumina was prepared by sol-gel method with aluminum nitrate and malic acid as raw materials. The effects of content of malic acid and polyvinylpyrrolidone (PVP) on sol spinnability were explored. The gel fibers with above 80 cm in length were obtained by mixing aluminum nitrate, malic acid and PVP on mass ratio of 10:3:1.5. Thermogravimetry-differential scanning calorimetry (TG-DSC), Fourier transform infrared (FTIR) spectrum, X-ray diffractometry (XRD), and scanning electron microscopy (SEM) were used to characterize the properties of the gel and ceramic fibers. The alumina fibers with a smooth surface and about 20 μm in diameter were obtained by sintering at 1 200 °C, and their main phase was indentified to be α-Al2O3.
Key words:
Al2O3; long fiber; sol-gel method; polyvinylpyrrolidone;
1 Introduction
Alumina is one of the most important materials because of its high strength and modulus, resistance to attacks from molten metals and non-oxide materials, chemical inertness in both oxidizing and reducing atmospheres up to 1 000 °C, and good electrical insulation [1]. Alumina also has high melting point (tm> 2 040 °C) and low thermal conductivity (10-18 W/(m?K)) [2]. An important potential application of alumina is as fiber reinforcement of metals, ceramics and resins [3-6].
Main processes for the manufacture of ceramic fibers can be classified as melt-spinning process and sol-gel spinning process [7]. Usually, the melt-spinning method was adopted for the synthesis of ceramic fibers with low melting point. So, the method was not suitable for the preparation of alumina fibers.
The preparation of short alumina fibers has been reported [8-10]. CHANDRADASS et al [8] prepared alumina short fibers by sol-gel process using aluminium- triisopropoxide (AIP) as the start materials. But, the AIP is expensive, and the process is limited in applications. SHOJAIE-BAHAABAD et al [9] synthesized composite fibers (YAG/Al2O3) from an aqueous solution of aluminum powder, aluminum chloride hexahydrate and yttrium oxide by sol-gel method. WANG et al [10] prepared alumina nanofibers by hydrolyzing aluminum nitrate in the presence of hexamethylenetetramine (HMTA) followed by the supercritical fluid drying (SCFD) process. And the δ-Al2O3 nanofibers were successfully synthesized, with diameter of 2 nm and length of 50 nm. The long quartz fibers have been produced by some factories, such as Mingda Co. Ltd, Jingzhou, China. But, the preparation processes of long alumina fibers have not observed in relevant reports. It is desirable to fabricate the long fibers of alumina using the Al source in low cost and distilled water as solvent with high fibres quality. In the present work, long alumina fibers are prepared by sol-gel method with aluminum nitrate (AN) and malic acid (MA) as raw materials, polyvinylpyrrolidone (PVP) as spinning additive. The process, crystallization phase and surface morphology are also investigated in detail.
2 Experimental
2.1 Preparation of samples
The starting materials used were AN (Chemically pure grade, Xi’an Reagent Factory, Xi’an, China), MA (Chemically pure grade, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) and PVP (Chemically pure grade, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China).
The alumina fibers were prepared in the processing steps shown in Fig. 1. The alumina sol was prepared by mixing H2O, aluminum nitrate and malic acid, followed by heating in water bath (80 °C). And, a proper amount of water and spinning additive (PVP) were added into the alumina gel. Then, the precursor solution was concentrated to obtain spinning sol in water bath (60 °C). The sol fibers were prepared by pulling a thin glass rod (diameter of about 5 mm, length of about 100 mm) slowly from the sol after immersing. Then, the sol fibers were dried at 60 °C for 24 h in an oven. The gel fibers were then sintered at 800, 1 000 and 1 200 °C for 1 h, respectively, with a heating rate of 1 °C/min.
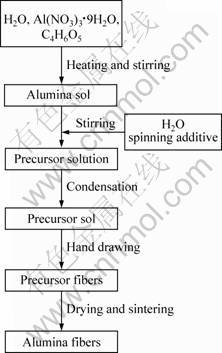
Fig. 1 Schematic view of production route for alumina fibers
2.2 Characterization techniques
For the gel fibers, thermal behaviors were measured by TG-DSC instruments (SDT Q600, TA Instrument, American) with a heating rate of 10 °C/min in flowing N2. Fourier transform infrared (FTIR) spectra were recorded on an infrared spectrometer (6700, Nicolet Magna, American) with the samples as KBr pellets. X-ray diffraction analysis was carried out on an X-ray diffractometer (D/max2400, Rigaku, Japan) using Cu Kα radiation, and a step width of 0.05 (°)/s. Morphologies of heat-treated fibers were characterized by scanning electron microscope (S-2700, Hitachi, Japan). All tests were done at room temperature.
3 Results and discussion
Alumina sol was prepared by synthesis and hydrolysis reaction which took place between aluminum nitrate and malic acid in aqueous solution during the stirring and heating. The main chemical reactions may be simplified as the following equations though the actual reactions are complex [11-12]:
Synthesis:
3C4H6O5+2Al(NO3)3→Al2(C4H4O5)3+6HNO3 (1)
HNO3→0.5H2O+0.25O2↑+NO2↑ (2)
Hydrolysis:
Al2(C4H4O5)3+6H2O→2Al(OH)3+3C4H6O5 (3)
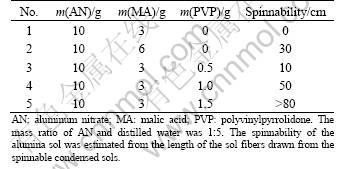
The macroscopic properties of the precursor sols prepared with different amounts of malic acid or spinning additive are listed in Table 1. The spinnability of sol was affected by the content of malic acid or spinning additive. When the malic acid amounted to 6 g in the sol, the sol was spinnable because almost all aluminum nitrate was involved in the reaction to generate aluminum malate. When aluminum malate solution was condensed, hydrolysis and condensation polycondensation could occur. After a concentrating process in water bath (60 °C), a spinnable sol was obtained, with the linear molecular chains. The mainly condensation polycondensation reactions can be simplified as the following equation [9]:
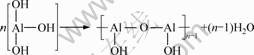 (4)
(4)
Moreover, the hydroxyl groups and carboxyl groups of malic acid could react with the aluminosilicate sol and an organic–inorganic hybrid structure was formed [7]. These overall reactions can be written as:
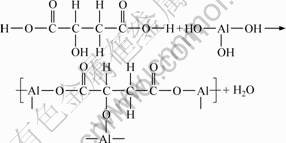 (5)
(5)
Table 1 Effect of MA and PVP on properties of precursor sols
Long alumina fibers can be obtained only by adding the spinning additive (e.g. PVP) because Al ions or particles would coordinate with N or O ions in PVP. The reactions can be written as [13]:
 (6)
(6)
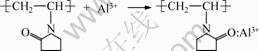 (7)
(7)
Otherwise, if too much organic acids remained, the densification of ceramic fibers would be delayed and microstructural defects would be generated during calcinations [14]. So, PVP was added to decrease the content of malic acid. When the content of PVP increased, the spinnability also increased in the sol 3 to 5. The sol 5 was suitable for fibers preparation because long fibers could be obtained.
The TG-DSC curves of the precursor gel fibers are shown in Fig. 2 with a heating rate of 10 °C/min. The DSC curve of the gel fibers exhibited two endothermic peaks at about 100 °C and 370 °C, and two exothermic peaks at about 725 °C and 972 °C. The endothermic peaks are assigned to the evaporation of the adsorbed water, and the decomposition of organic precursor in the gel fibers, whereas the two exothermic peaks are assigned to the amorphous/γ-Al2O3 transformation and α-Al2O3 phase crystallization, respectively [15]. The TG curve of the gel fibers shows a mass loss around 60% at 800 °C, while almost no further mass loss appeared with further increasing the temperature.

Fig. 2 TG and DSC curves of alumina precursor gel fibers
The FTIR spectrum of precursor gel fibers is shown in Fig. 3. As can be seen, the bands at 3 440 cm-1 and 1 170 cm-1 are assigned to the O—H stretching modes and bending mode of adhesive and constitution water as well as malic acid and spinning additive, respectively. The band at 2 550 cm-1 is assigned to the O—N stretching mode of nitric acid. The bands at 1 710 cm-1 and 476 cm-1 are assigned to the C=O stretching mode and bending mode, respectively. The band at 910 cm-1 is assigned to the C—C stretching mode. The bands at 1 360 cm-1 and 820 cm-1 may be assigned to the C—O stretching mode and bending mode, respectively. As can be seen, a little of nitric acid was present in the samples.
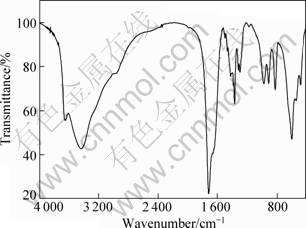
Fig. 3 FT-IR spectrum of precursor gel fibers
As shown in Fig. 3, the band observed at 1 420 cm-1 corresponds to Al—OH bonding mode [8]. The stretching modes of Al—O—Al linkages are observed at 600 cm-1 and 820 cm-1 [16]. When the precursor solution was condensed, the hydrolysis and condensation polycondensation could occur. So, the stretching modes of Al—O—Al linkages are observed.
The XRD patterns of gel fibers sintered at 800, 1 000, and 1 200 °C are shown in Fig. 4. Only amorphous and γ-Al2O3 phases were present when the fibers were sintered at 800 °C. α-Al2O3 phase was observed in the samples sintered at 1 000 °C, while main α-Al2O3 phase at 1 200 °C. From the DSC and XRD results, it can be concluded that α-Al2O3 phase crystallization of the fibers occurred at temperature of about 972 °C.
It has been shown that phase development during crystallization of amorphous alumina takes place through the following route: amorphous→etae(η)→gammae(γ)→deltae(δ)→thetae(θ)→alphae(α)
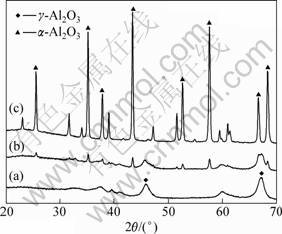
Fig. 4 XRD patterns of precursor gel fibers heated at 800 (a), 1 000 (b), and 1 200 °C (c) for 1 h
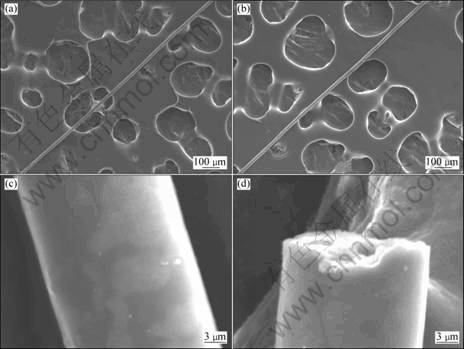
Fig. 5 SEM images of alumina precursor gel fibers heated at 1 200 °C for 1 h: (a), (b), (c) Surface of fibers; (d) Cross-section of fibers
Upon heating, the θ-Al2O3 undergoes a reconstructive transformation by nucleation and growth, where the oxygen atoms rearrange into a hexagonal close packed structure to form thermodynamically stable α-Al2O3 [17]. During the reconstructive transformation from theta to alpha alumina, there is a specific volume reduction (28.6-25.6 cm3/mol), due to the difference in theoretical density (3.6-3.986 g/cm3) [18]. A low intrinsic nucleation density results in a large spacing between nucleation events and the formation of micrometered, single crystal α-Al2O3 grains with dendritic protrusions surrounded by continuous pore channels [18]. The resultant vermicular microstructure requires sintering at a temperature higher than 1 600 °C to obtain high densities [19]. The sintering temperature can be decreased by adding low melting point phase (e.g. SiO2, B2O3, TiO2).
SEM micrographs of alumina fibers sintered at 1 200 °C are shown in Fig. 5. The fibers had a diameter of about 20 μm, with a uniform diameter. The diameter of fibers was influenced by viscosity and surface tension of spinning sol, speed of hand drawing and so on. The further research about these factors is carrying on.
4 Conclusions
1) Long alumina fibers were prepared by sol-gel method when the spinning sol was prepared by mixing aluminum nitrate, malic acid and polyvinylpyrrolidone with a mass ratio of 10:3:1.5. The gel fibers were obtained with above 80 cm in length.
2) The main phase was amorphous and γ-Al2O3 phase when the gel fiber was sintered at 800 °C. The main phase of fibers was α-Al2O3 by sintering at 1 200 °C. The alumina fibers with a smooth surface and about 20 μm in diameter were obtained by sintering at 1 200 °C.
References
[1] ZHU L, HUANG Q, LIU W. Synthesis of plate-like α-Al2O3 single-crystal particles in NaCl-KCl flux using Al(OH)3 powders as starting materials [J]. Ceramics International, 2008, 34(7): 1729-1733.
[2] SEDAGHAT A, TAHERI-NASSAJ E, NAGHIZADEH R. An alumina mat with a nano microstructure prepared by centrifugal spinning method [J]. Journal of Non-crystalline Solids, 2006, 352(26-27): 2818-2828.
[3] OKABE T, NISHIKAWA M, TAKEDA N, SEKINE H. Effect of matrix hardening on the tensile strength of alumina fiber-reinforced aluminum matrix composites [J]. Acta Materialia, 2006, 54(9): 2557-2566.
[4] RIEHEMANN W, TROJANOVA Z, MIELCZAREK A. Fatigue in magnesium alloy AZ91-γ-alumina fiber composite studied by internal friction measurements [J]. Procedia Engineering, 2010, 2(21): 2151-2160.
[5] NASKAR M, BASU K, CHATTERJEE M. Sol-gel approach to near-net-shape oxide–oxide composites reinforced with short alumina fibres—The effect of crystallization [J]. Ceramics International, 2009, 35(8): 3073-3079.
[6] BAO Y H, NICHOLSON P S. AlPO4-coated mullite/alumina fiber reinforced reaction-bonded mullite composites [J]. Journal of the European Ceramic Society, 2008, 28 (16): 3041-3048.
[7] ZHANG Y, DING Y, GAO J, YANG J. Mullite fibres prepared by sol-gel method using polyvinyl butyral [J]. Journal of the European Ceramic Society, 2009, 29(6): 1101-1107.
[8] CHANDRADASS J, BALASUBRAMANIAN M. Sol-gel processing of alumina fibres [J]. Journal of Materials Processing Technology, 2006, 173 (3): 275-280.
[9] SHOJAIE-BAHAABAD M, TAHERI-NASSAJ E, NAGHIZADEH R. An alumina-YAG nanostructured fiber prepared from an aqueous sol-gel precursor: Preparation, rheological behavior and spinnability [J]. Ceramics International, 2008, 34(8): 1893-1902.
[10] WANG J Q, WANG Y Z, QIAO M H, XIE S H, FAN K N. A novel sol-gel synthetic route to alumina nanofibers via aluminum nitrate and hexamethylenetetramine [J]. Materials Letters, 2007, 61(28): 5074-5077.
[11] ZHANG R, HONG Q, YANG J, ZHANG H, BLACKBURNB G M, ZHOU Z. Syntheses, spectroscopies and structures of zinc complexes with malate [J]. Inorganica Chimica Acta, 2009, 362(8): 2643-2649.
[12] VENTURINI-SORIANO M, BERTHON G. Aluminum speciation studies in biological fluids (Part 7): A quantitative investigation of aluminum(III)-malate complex equilibria and their potential implications for aluminum metabolism and toxicity [J]. Journal of Inorganic Biochemistry, 2001, 85(2-3): 143-154.
[13] WANG H S, QIAO X L, CHEN J G, WANG X J, DING S Y. Mechanisms of PVP in the preparation of silver nanoparticles [J]. Materials Chemistry and Physics, 2005, 94(2-3): 449-453.
[14] VENKATESH R, RAMANAN S R. Effect of organic additives on the properties of sol-gel spun alumina fibres [J]. Journal of the European Ceramic Society, 2000, 20(14-15): 2543-2549.
[15] LI J, PAN Y, XIANG C, GE Q, GUO J. Low temperature synthesis of ultrafine α-Al2O3 powder by a simple aqueous sol-gel process [J]. Ceramics International, 2006, 32(5): 587-591.
[16] PADMAJ P, ANILKUMAR G M, MUKUNDAN P, ARULDHAS G, WARRIER K G K. Characterisation of stoichiometric sol–gel mullite by Fourier transform infrared spectroscopy [J]. International Journal of Inorganic Materials, 2001, 3(7): 693-698.
[17] NORDAHL C S, MESSING G L. Thermal analysis of phase transformation kinetics in α-Al2O3 seeded boehmite and γ-Al2O3 [J]. Thermochimica Acta, 1998, 318(1-2): 187-199.
[18] NORDAHL C S, MESSING G L. Sintering of α-Al2O3-seeded nanocrystalline γ-Al2O3 powders [J]. Journal of the European Ceramic Society, 2002, 22(4): 415-422.
[19] LI J, SUN X. Synthesis and sintering behavior of a nanocrystalline α-Al2O3 powder [J]. Acta Mater, 2000, 48(12): 3103-3112.
苹果酸溶胶-凝胶法制备氧化铝长纤维
谭宏斌,郭从盛
陕西理工学院 材料科学与工程学院,汉中 723003
摘 要:用硝酸铝、苹果酸为原料,用溶胶-凝胶法制备氧化铝前驱体溶胶。研究了苹果酸、聚乙烯吡咯烷酮(PVP)的加入量对溶胶可纺性能的影响。研究发现:当硝酸铝、苹果酸、PVP的质量比为10:3:1.5时,得到的凝胶纤维长度大于80 cm。用热重-差示扫描(TG-DSC)、傅里叶转换红外线光谱(FTIR)、X射线衍射(XRD)、扫描电镜(SEM)分析方法对凝胶纤维和陶瓷纤维进行了表征。凝胶纤维的长度大于80 cm。凝胶纤维在1 200 °C煅烧1 h后,物相为α-Al2O3,得到的氧化铝纤维直径均匀、表面光滑,纤维直径为20 μm。
关键词:Al2O3;长纤维;溶胶-凝胶法;聚乙烯吡咯烷酮
(Edited by YANG Hua)
Foundation item: Project (2010K10-21) supported by the Natural Science Foundation of Shaanxi Province, China
Corresponding author: TAN Hong-bin; Tel: +86-916-2291082; E-mail: hb-t@163.com
DOI: 10.1016/S1003-6326(11)60897-2


