Trans. Nonferrous Met. Soc. China 23(2013) 1395-1402
Influence of pH value and chelating reagent on performance of Li3V2(PO4)3/C cathode material
Wei XIANG1, Yan TANG1, Yan-ying WANG1, Ben-he ZHONG1, Wei-mao FANG1, Heng LIU2, Xiao-dong GUO1
1. School of Chemical Engineering, Sichuan University, Chengdu 610065, China;
2. School of Materials Science and Engineering, Sichuan University, Chengdu 610065, China
Received 3 May 2012; accepted 26 December 2012
Abstract:
The Li3V2(PO4)3/C composite cathode material was synthesized via sol-gel method using three different chelating agents (citric acid, salicylic acid and polyacrylic acid) at pH value of 3 or 7. The crystal structure, morphology, specific surface area and electrochemical performance of the prepared samples were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge/discharge test. The results show that the effects of pH value on the performance of the prepared materials are greatly related to the chelating agents. With salicylic acid or polyacrylic acid as the chelating reagent, the structure, morphology and electrochemical performance of the samples are greatly influenced by the pH values. However, the structure of the materials with citric acid as the chelating agent does not change as pH value changes, and the materials own uniform particle size distribution and good electrochemical performance. It delivers an initial discharge capacity of 113.58 mA·h/g at 10C, remaining as high as 108.48 mA·h/g after 900 cycles, with a capacity retention of 95.51%.
Key words:
Li3V2(PO4)3; cathode material; sol-gel method; chelating agents; pH value;
1 Introduction
The development of new energy sources, especially the lithium-ion batteries, has attracted tremendous interest due to the depletion of fossil energy and environmental issues. Cathode material which accounts for more than 30% of the total cost is the key to the development of lithium-ion battery. Currently, LiCoO2 is widely used as cathode material in commercial lithium-ion batteries, but its high cost and toxicity inhibit its use in large-scale, so more efforts have been made to study new cathode materials. Li3V2(PO4)3 is a high promising material proposed as a cathode for higher voltage Li-ion batteries because of its good ion mobility, high reversible capacity and high operating voltage [1,2]. The reversible cycling of all three lithium ions from Li3V2(PO4)3 corresponds to a high theoretical capacity of 197 mA·h/g [3,4]. Combining with its high safety, Li3V2(PO4)3 has been proposed to be a potential candidate of cathode materials for Li-ion batteries.
Many methods are applied to synthesizing Li3V2(PO4)3 cathode materials, including conventional solid-state synthesis [5-7], hydrothermal [8,9], sol-gel method [10-13], microwave solid-state synthesis method [14], and other soft chemistry methods [15-17], etc. Among them sol-gel method allows reactants to mix at the atomic or molecular level. Meanwhile, enormous advantages such as lower calcination temperature, shorter sintering time and smaller particle size are the important considerations. Thus, sol-gel method is promising to synthesize material of higher purity and smaller particle sizes.
TANG et al [18,19] reported that the conditions of solution during the sol-gel process greatly influenced the electrochemical performance of the samples. In this study, the pH value of solution was found to have great impact on the performance of the materials. Moreover, the impact factor of pH value varies with the type of chelating agents used. The physical properties and electrochemical performance of the synthesized materials were discussed in detail.
2 Experimental
2.1 Materials and preparation
The samples were prepared by the sol-gel method. LiOH·H2O (99%), NH4VO3 (99%), H3PO4 (85%) at stoichiometric ratio were used as raw materials. Citric acid, salicylic acid and polyacrylic acid were used as carbon sources and chelating reagents. Firstly, LiOH·H2O and NH4VO3 were dissolved in deionized water at room temperature. H3PO4 and one of the chelating reagents were then added into the solution. Finally, a certain amount of NH3·H2O was added to adjust the pH value of solution. This solution was constantly stirred at 80 °C for 2 h, and then the excess solvent was removed by vacuum distillation. The resulting gel precursor was dried in a vacuum oven at 90 °C for 15 h. After being dried, the precursors were decomposed in a tube furnace at 350 °C for 4 h, and then heated at 700 °C for 6 h under flowing argon. The final product was a black powder. The samples synthesized with citric acid, salicylic acid and polyacrylic acid under pH value of 3 were marked as A1, B1 and C1, respectively, and A2, B2 and C2, respectively for pH value of 7.
2.2 Characterization
The carbon content was verified by analytical instrument (CS-902, Wanlianda Xinke, Beijing, China). The residual carbon contents of all the samples were 2%-3% (mass fraction). The crystalline structure of each product was analyzed by X-ray diffractometer (XRD, D/max-rB, Rigaku, Cu Kα radiation) (λ=1.5046 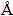 ) operated at 40 kV and 40 mA. The particle morphology and particle size of the Li3V2(PO4)3 powders were observed on a scanning electron microscope (SEM, SPA400 Seiko Instruments). The particle size distribution was estimated on a laser particle size distribution tester (JL-1155).
) operated at 40 kV and 40 mA. The particle morphology and particle size of the Li3V2(PO4)3 powders were observed on a scanning electron microscope (SEM, SPA400 Seiko Instruments). The particle size distribution was estimated on a laser particle size distribution tester (JL-1155).
2.3 Electrochemical measurements
The electrochemical properties of Li3V2(PO4)3 were evaluated in CR2032 coin cells, which consisted of a LVP/C working electrode and a lithium foil counter electrode separated by a Celgard 2300 microporous membrane. The cathode was fabricated by pressing a mixture (in mass fraction) of 80% active material, 13% acetylene black (conducting additive) and 7% PVDF (binder) onto an Al foil. The anode was lithium foil and the electrolyte was 1 mol/L LiPF6 solution in ethylene carbonate (EC):dimethyl carbonate (DMC) (1:1 in volume ratio). Galvanostatic charging/discharging tests were conducted at different current densities in the voltage range of 3.0-4.5 V with a battery test system (Neware BTS-610). Cyclic voltammetry (CV) was performed by a LK9805 electrochemical analyser between 3.0 and 4.5 V at a scanning rate of 0.1 mV/s. Electrochemical impedance spectroscopy (EIS) was conducted at a frequency range of 10 mHz-100 kHz with a vibration of 5 mV using a Zahner electrochemical work station.
3 Results and discussion
3.1 Sample characterization
Figure 1 shows the X-ray diffraction patterns of the Li3V2(PO4)3/C composites synthesized with different chelating reagents under various pH values. It can be seen that at pH=3, the characteristic peaks of all the samples are sharp and without any impurity peaks. There is also no additional diffraction peak related the carbon, which can be attributed to the low content (shown in Table 1) or amorphous form. However, when pH values are adjusted to 7, the XRD patterns are different because of different chelating reagents. Compared with sample A2, there are some impurities peaks displayed on the XRD patterns of samples B2 and C2. What’s more, the intensities of diffraction peaks of sample C2 are much weaker than those of the other two samples. Table 1 shows the crystallinity and grain sizes of all the samples calculated by Jade 5.0 software. It can be seen that samples B2 and C2 have smaller crystallinity than the others, which corresponds to the results of characteristic peaks intensity. Sample C2 has the lowest crystallinity and the smallest grain sizes, indicating that the cell of sample C2 does not grow well, which may be quite disadvantageous to its electrochemical properties.
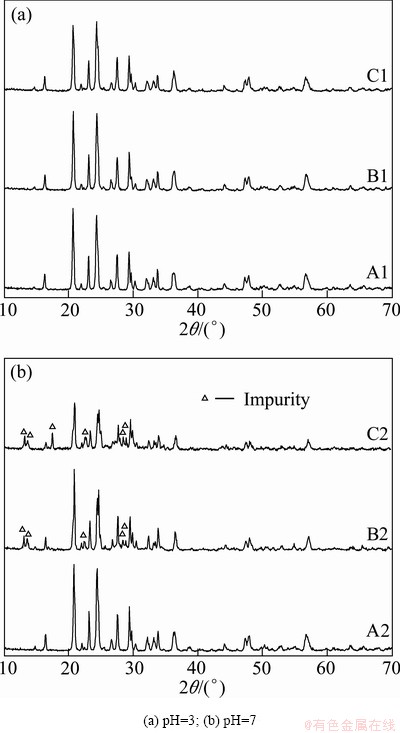
Fig. 1 XRD patterns of all samples
Table 1 Physical parameters of Li3V2(PO4)3/C samples

Figure 2 shows the SEM images of all the samples, and much difference in the morphologies with the pH value changing can be observed clearly. Samples A1 and A2 prepared with critic acid as chelating reagents have small particle size. When the pH value turns to 7, the materials have more uniform particle size distribution and small particles agglomerated. The particle size distribution for sample A2 is shown in Fig. 3. The diameters at 10%, 50%, 75% and 99% cumulative volume for the particles are 0.195, 0.528, 1.019 and 2.853 μm, and the average size 0.747 μm, which is consistent with the results of SEM. Sample B2 exhibits a similar particle shape, but a smaller particle size compared with B1. The morphology of sample C2 is different from that of sample C1. The particles are block and distributed in 2-3 μm with a smooth surface, which does not favour lithium-ion diffusion. The carbon content of sample C2 is as high as 5.39%, but the particles are also big. The impurities in sample C2 may be the main reason resulting in big particles.
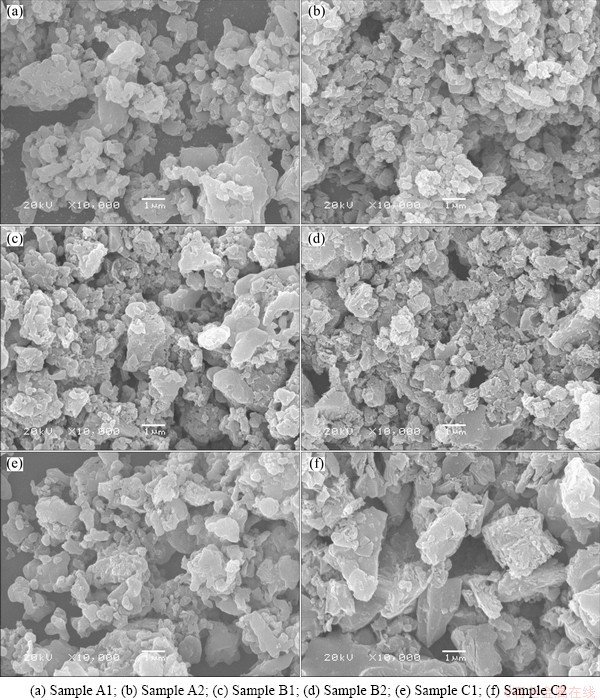
Fig. 2 SEM images of samples

Fig. 3 Particle size distribution of sample A2
Based on XRD and SEM results, it is obvious that the stability of chelating reagents is so much related to the pH value. Compared to salicylic acid and polyacrylic acid, critic acid has the highest stability. Since critic acid (H3Cit) is a polybasic acid, multi-stage ionization happens according to different pH values of the solution. Therefore, the ions in the solution are various under different pH values. When the pH value is 3, the amount of H3Cit and H2Cit- ions is the largest, and the chelating reactions can occur smoothly; when pH value is increased to 7, H2Cit- and HCit2- ions are dominated, which are helpful to the chelating reactions. However, the other two organic acids have weaker chelation. When pH value is as high as 7, the vanadate will separately form as precipitation and result in big particles and impurity, which may have a notable effect on the electrochemical performance of Li3V2(PO4)3.
3.2 Electrochemical properties
Figure 4 shows the initial discharge curves of all the samples. It can be seen that all the samples display typical voltage profiles of Li3V2(PO4)3 containing three discharge flat plateaus at a lower rate. However, with the increase of current density, the differences of polarization for all the samples are greater. Samples A1(pH=3) and A2 (pH=7) with critic acid as chelating reagent show lower polarization and good rate capacity (Fig. 4(a) and (b)). The two samples exhibit the similar initial discharge capacities of 123.6 mA·h/g and 121.5 mA·h/g at 0.1C. Sample A2 exhibits higher rate capacity. The initial discharge capacities of sample A2 at 0.1C, 0.2C, 0.5C, 1C, 3C, 5C, 10C and 20C are 121.5, 117.8, 116.7, 115.9, 114.5, 114.0, 112.7 and 110.8 mA·h/g, respectively; however, those of sample A1 are 123.6, 120.8, 117.2, 116.9, 113.2, 110.6, 104.0 and 92.9 mA·h/g, respectively. With salicylic acid as chelating reagent, the polarizations become higher, the voltage of the flat plateaus decreases fast with the increase of current density (Fig. 4(c)). Although sample B2 (pH=7) displays a higher initial discharge capacity (103.7 mA·h/g) than sample B1 (pH=3, 89.7 mA·h/g), they both exhibit bad rate capability, and the capacities at 20C are only about 60 mA·h/g. When using polyacrylic acid as chelating reagent, the initial discharge capacities of the two samples are lower than those of the others because the carbon content is as high as 5.3%. So, the active material in Li3V2(PO4)3/C composite material is lower, resulting in lower initial discharge capacity. From Fig. 4(e), it can be seen that the pH value of solution has great influence on the electrochemical properties. Sample C1 has lower polarization and the discharge capacities decrease slightly with the increase of current density; however, sample C2 displays very bad electrochemical properties, and the initial discharge capacity is as low as 67.3 mA·h/g at 0.1C, and only 28.5 mA·h/g under 20C.
Figure 5 displays the cycling performance of all the samples at different discharge rates between 3.0 and 4.5 V. As can be seen, samples A1and A2 have the best electrochemical performance. Their discharge capacities are much higher than those of the others, which maintain 94.8 and 110.8 mA·h/g at a high rate of 20C. Samples B1 and B2 show a severe decline in capacity during cycling, specifically sample B2. The bad rate performance of sample B2 may be attributed to its low purity. Sample C1 shows better electrochemical performance and the worst capacity happens to sample C2. Combined with the results of XRD and SEM, the low discharge capacities may be attributed to the poor crystallinity, low purity and big particle size of the sample.
Sample A2 can not only supply large capacity under high rate conditions, but also maintain good cycling stability. As shown in Fig. 6, under a high rate of 10C, a capacity of 113.58 mA·h/g is delivered. After 900 cycles it is also as high as 108.48 mA·h/g, with a capacity retention of 95.51%. The inset figure shows the charge/discharge curves. There are three flat plateaus on the discharge curves, which nearly keeps the same after 900 cycles. The promising electrochemical performance of the as-prepared Li3V2(PO4)3/C composite can be attributed to the small particle size and large specific area, which are beneficial for the lithium extraction and insertion reactions under high discharge current. According to the electrochemical performance presented above, the as-prepared Li3V2(PO4)3/C composite cathode material is very attractive to develop high power lithium-ion batteries for electric vehicles (EVs) or hybrid electric vehicles (HEVs).

Fig. 4 Initial discharge curves of all samples at different discharge rates
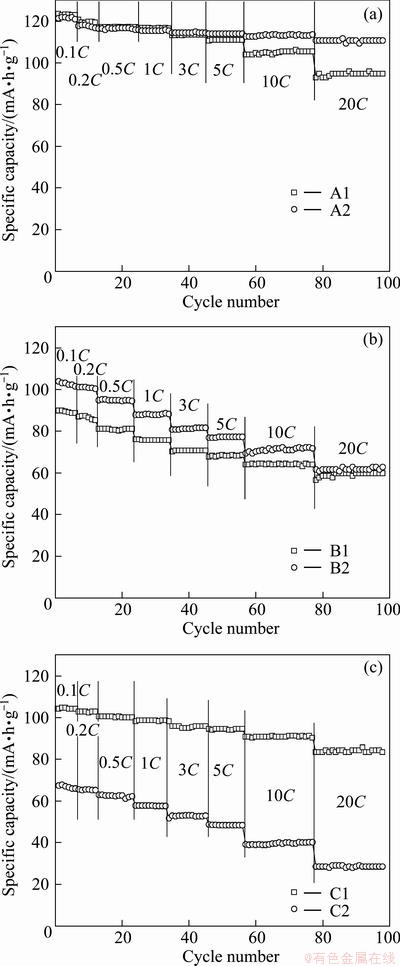
Fig. 5 Cycling performance of all samples at different discharge rates
Figure 7 shows the CV curves of sample A2 from 3.0 V to 4.5 V at a scanning rate of 0.10 mV/s. It exhibits typical oxidative peaks near at 3.62, 3.71, and 4.12 V (vs Li/Li+) as well as reductive peaks 3.55, 3.63, and 4.01 V (vs Li/Li+), respectively, which correspond to the charge/discharge curves. Since only two Li+ ions are removed, all of the three current peaks are assigned to the V3+/V4+ redox couple. The redox peaks are sharp and the redox capacities (peak areas) ratio is close to 1, indicating a strong reversibility of the electrode reaction. In addition, the separations of redox peaks are small, and the maximum one is only 0.11 V, which also shows a good reversibility of electrode reaction, indicating that the material has good cycling performance.
Figure 8 presents the Nyquist plots of all the samples measured at the open-circuit voltage after galvanostatic charging/discharging tests at 20C. The spectra of samples show an intercept at high frequency, followed by a depressed semicircle in the high-middle frequency region, and a straight line in the low frequency region. Refinements of the diagrams were conducted by using the Zview2 program. An equivalent circuit has been built as shown in Figs. 8(a) and (b). In the circuit, the Re represents the solution resistance of the electrolyte; Rct and CPE1 stand for the charge-transfer resistance and double-layer capacitance, respectively; W represents the diffusion-controlled Warburg impedance. The parameters of the equivalent circuit are shown in Table 2. It is found that the Re is similar for all the samples at 2-5 Ω. Rct values of sample A1 (66.1 Ω) and A2 (64.0 Ω) are much lower than the others after about 100 cycles, indicating that the two samples have better cycle performance. For the other samples, the Rct values are much larger, especially the one has changed the pH value to 7, which may be attributed to the impurity in the materials increasing the impedance remarkably.
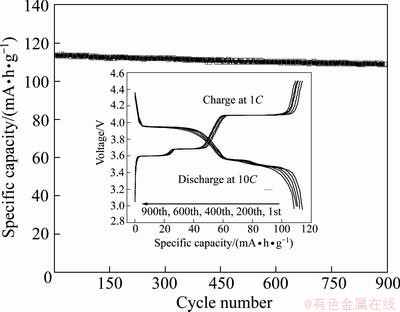
Fig. 6 Cycling performance of sample A2 at rate of 10C

Fig. 7 Cyclic voltammograms of sample A2
Table 2 Parameters obtained from equivalent circuit fitting of experiment data
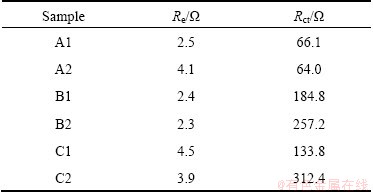
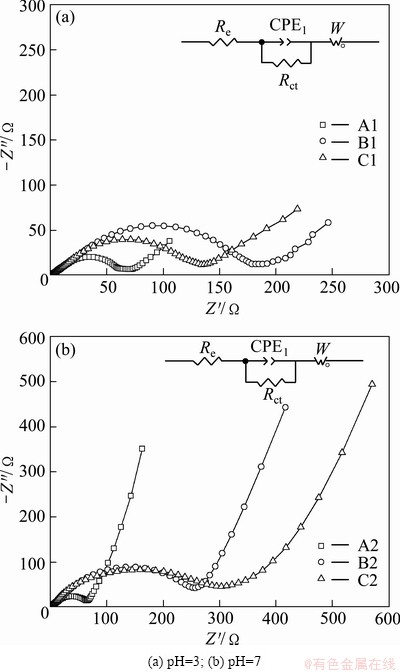
Fig. 8 EIS curves of all samples
4 Conclusions
1) Li3V2(PO4)3/C composite cathode material was synthesized via sol-gel method using three different chelating reagents (citric acid, salicylic acid and polyacrylic acid) under different pH values (3, 7).
2) XRD patterns and SEM images show that increasing the pH value is good for the samples with critic acid as chelating reagents; however, when salicylic acid or polyacrylic acid is used as chelating reagent, high pH value leads to impurity and big particles.
3) Electrochemical tests indicate that the samples with critic acid as chelating reagents have better electrochemical performance than the others, especially the one with pH value of 7 demonstrated a good rate performance and a stable long-term cycling capacity of 113.58 mA·h/g with capacity retention of 95.51% at discharge rate of 10C up to 900 cycles.
References
[1] FU Peng, ZHAO Yan-ming, DONG You-zhong, HOU Xing-mei. Synthesis of high tap density Li3V2(PO4)3 cathode materials using mixed lithium precursors [J]. Journal of Physics and Chemistry of Solids, 2010, 71(3): 394-399.
[2] LI Li, LI Guo-hua, WANG Shi-quan, FENG Chun-qi. Synthesis and electrochemical characteristics of cathode materials Li3V2(PO4)3 [J]. Chinese Journal Inorganic Chemistry, 2010, 26(1): 126-131. (in Chinese)
[3] QIAO Y Q, TU J P, WANG X L, ZHANG D, XIANG J Y, MAI Y J, GU C D. Synthesis and improved electrochemical performances of porous Li3V2(PO4)3/C spheres as cathode material for lithium-ion batteries [J]. J Power Sources, 2011, 196(18): 7715-7720.
[4] RUI X H, YESIBOTATI N, CHEN C H. Synthesis and characterization of carbon-coated Li3V2(PO4)3 cathode materials with different carbon sources [J]. J Power Sources, 2011, 196(4): 2279-2282.
[5] WANG Jia-wei, LIU Jing, YANG Gui-ling, ZHANG Xian-fa, YAN Xue-dong, PAN Xiu-mei, WANG Rong-shun. Electrochemical performance of Li3V2(PO4)3/C cathode material using a novel carbon source [J]. Electrochimica Acta, 2009, 54(26): 6451-6454.
[6] RUI X H, LI C, CHEN C H. Synthesis and characterization of carbon-coated Li3V2(PO4)3 cathode materials with different carbon sources [J]. Electrochimica Acta, 2009, 54(12): 3374-3380.
[7] WANG Li-juan, ZHOU Xue-chou, GUO Yong-lang. Synthesis and performance of carbon-coated Li3V2(PO4)3 cathode materials by a low temperature solid-state reaction [J]. J Power Sources, 2010, 195(9): 2844-2850.
[8] CHANG Cai-xian, XIANG Jiang-feng, SHI Xi-xi, HAN Xiao-yan, YUAN Liang-jie, SUN Ju-tang. Hydrothermal synthesis of carbon-coated lithium vanadium phosphate [J]. Electrochimica Acta, 2008, 54(2): 623-627.
[9] LIU Hao-wen, CHENG Cui-xia, HUANG Xin-tang, LI Jin-lin. Hydrothermal synthesis and rate capacity studies of Li3V2(PO4)3 nanorods as cathode material for lithium-ion batteries [J]. Electrochimica Acta, 2010, 55(28): 8461-8465.
[10] ZHU X J, LIU Y X, GENG L M, CHEN L B. Synthesis and performance of lithium vanadium phosphate as cathode material for lithium ion batteries by a sol-gel method [J]. J Power Sources, 2008, 184(2): 578-582.
[11] CHEN Ying-hua, ZHAO Yan-ming, AN Xing-ning, LIU Jian-min, DONG You-zhong, CHEN Ling. Preparation and electrochemical performance studies on Cr-doped Li3V2(PO4)3 as cathode materials for lithium-ion batteries [J]. Electrochimica Acta, 2009, 54(24): 5844-5850.
[12] ZHAI Jing, ZHAO Min-shou, WANG Dan-dan, QIAO Yu-qing. Effect of MgO nanolayer coated on Li3V2(PO4)3/C cathode material for lithium-ion battery [J]. J Alloys and Compounds, 2010, 502(2): 401-406.
[13] ZHAI Jing, ZHAO Min-shou, WANG Dan-dan. Effect of Mn-doping on performance of Li3V2(PO4)3/C cathode material for lithium ion batteries [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(3): 523-528.
[14] YANG Gang, LIU Hai-dong, JI Hong-mei, CHEN Zhong-zhong, JIANG Xue-fan. Microwave solid-state synthesis and electrochemical properties of carbon-free Li3V2(PO4)3 as cathode materials for lithium batteries [J]. Electrochimica Acta, 2010, 55(8): 2951-2957.
[15] WANG Lian, LI Xiang, JIANG Xian-quan, PAN Fu-sheng, WU Feng. Wet coordination method to prepare carbon-coated Li3V2(PO4)3 cathode material for lithium ion batteries [J]. Solid State Sciences, 2010, 12(7): 1248-1252.
[16] AI Deng-jun, LIU Wei-fang, TANG An-ping, DUAN Hao, LIU Kai-yu. Preparation and electrochemical properties of monoclinic Li3V2(PO4)3/C composites [J]. Chinese Journal Inorganic Chemistry, 2009, 25(10): 1717-1723. (in Chinese)
[17] HUANG J S, YANG L, LIU K Y. One-pot syntheses of Li3V2(PO4)3/C cathode material for lithium ion batteries via ascorbic acid reduction approach [J]. Materials Chemistry and Physics, 2011, 128(3): 470-474.
[18] TANG Yan, ZHONG Ben-he, GUO Xiao-dong, LIU Heng, ZHONG Yan-jun, NIE Xiang, TANG Hong. Effects of mixed solvents on the high-rate performance of Li3V2(PO4)3/C prepared by sol-gel method [J]. Acta Phys-Chim Sin, 2011, 27(4): 869-874.
[19] TANG Yan, GUO Xiao-dong, NIE Xiang, ZHONG Yan-jun, ZHONG Ben-he, LIU Heng, WEN Jia-jie. Synthesis and performance of Li3V2(PO4)3/C with different organic-water mixed solvents [J]. The Chinese Journal of Nonferrous Metals, 2012, 22(1): 179-186. (in Chinese).
pH值和螯合剂复合作用对正极材料Li3V2(PO4)3/C性能的影响
向 伟1,唐 艳1,王雁英1,钟本和1,方为茂1,刘 恒2,郭孝东1
1. 四川大学 化学工程学院,成都 610065;
2. 四川大学 材料科学与工程学院,成都 610065
摘 要:使用不同的螯合剂(柠檬酸、水杨酸和聚丙烯酸)在不同的pH值条件下,采用溶胶-凝胶法合成Li3V2(PO4)3/C正极材料。通过X射线衍射(XRD)、扫描电镜(SEM)、循环伏安(CV)、电化学阻抗谱(EIS)以及恒流充放电测试等方法, 研究材料的结构、形貌及电化学性能。结果表明,pH值对材料性能的影响与所采用的螯合剂有很大的关系。当采用水杨酸和聚丙烯酸为螯合剂时,升高pH值对材料的结构、形貌和电化学性能有较大的负面影响。而采用柠檬酸为螯合剂时,材料结构无变化且颗粒分布更均匀,高倍率放电性能和循环性能也得到改善,10C首次放电容量为113.58 mA·h/g,循环900次后容量保持率为95.51%。
关键词:Li3V2(PO4)3;正极材料;溶胶-凝胶法;螯合剂;pH值
(Edited by Hua YANG)
Foundation item: Project (2007BAQ01055) supported by the National Key Technology R&D Program of China; Project (2011SCU11081) supported by the Sichuan University Funds for Young Scientists, China; Project (20120181120103) supported by Ph. D. Programs Foundation of the Ministry of Education of China
Corresponding author: Xiao-dong GUO; Tel: +86-28-85406702; E-mail: xiaodong2009@scu.edu.cn
DOI: 10.1016/S1003-6326(13)62609-6
Abstract: The Li3V2(PO4)3/C composite cathode material was synthesized via sol-gel method using three different chelating agents (citric acid, salicylic acid and polyacrylic acid) at pH value of 3 or 7. The crystal structure, morphology, specific surface area and electrochemical performance of the prepared samples were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge/discharge test. The results show that the effects of pH value on the performance of the prepared materials are greatly related to the chelating agents. With salicylic acid or polyacrylic acid as the chelating reagent, the structure, morphology and electrochemical performance of the samples are greatly influenced by the pH values. However, the structure of the materials with citric acid as the chelating agent does not change as pH value changes, and the materials own uniform particle size distribution and good electrochemical performance. It delivers an initial discharge capacity of 113.58 mA·h/g at 10C, remaining as high as 108.48 mA·h/g after 900 cycles, with a capacity retention of 95.51%.


