
Effects of catalyst precursors on carbon nanowires by using ethanol catalytic combustion technique
CHENG Jin(程 进)1,2, ZOU Xiao-ping(邹小平) 1,2, LI Fei(李 飞) 1,2, ZHANG Hong-dan(张红丹) 1,2, REN Peng-fei(任鹏飞) 1,2
1.Research Center for Sensor Technology, Beijing Information Technology Institute, Beijing 100101, China;
2.Beijing Key Laboratory for Sensor, Beijing 100101, China
Received 10 April 2006; accepted 25 April 2006
Abstract:
Iron nitrate, nickel nitrate and cobalt nitrate were used as catalyst precursors to study their effects on carbon nanowires synthesized by ethanol catalytic combustion (ECC) process. The as-grown carbon nanowires were characterized by means of scanning electron microscopy, transmission electron microscopy and Raman spectroscopy. The results show that relatively uniform nanowires will be formed when the catalyst precursor is iron nitrate; while helical structure or disordered structure will be formed when the catalyst precursor is nickel nitrate or cobalt nitrate.
Key words:
carbon nanowires; ethanol catalytic combustion technique; catalyst precursor;
1 Introduction
Quasi-one-dimensional nanomaterial is key aspect in the emerging nanotechnology. However, large-scale and controlled synthesis is still limited because the morphology and microstructure of quasi-one-dimensionalnanomaterials could be affected by source materials[1], catalysts[2,3], substrates[4], carrier gas[5], and synthesis parameters[6,7]. It is essential to study the effects of various factors on synthesis of quasi-one-dimensional nanomaterials for controlled synthesis which is important to realize nanostructures or nanodevices for applications.
Carbon nanowires as quasi-one-dimensional nanomaterials have a similarly attractive property to carbon nanotubes, such as high aspect ratio, high conductivity, and high elastic modulus. So many synthesis approaches have been developed for synthesizing carbon nanowires, for instance, electrospinning[8], chemical vapor deposition[9]. However, current synthesis methods suffer from high cost, complex experimental setup, etc. In this paper, a simple synthesis approach for synthesizing carbon nanowires was developed. Iron nitrate, nickel nitrate and cobalt nitrate were used as catalyst precursors to study the effects of catalyst precursors on carbon nanowires synthesized by ethanol catalytic combustion (ECC) process.
2 Experimental
Carbon nanowires were synthesized on copper plate by ECC process. The catalyst precursors were saturation solution of iron nitrate, nickel nitrate and cobalt nitrate, respectively. The catalyst precursor solution was applied to copper plate that was ultrasonically washed in acetone for several minutes. Then the copper substrates were placed in an inner flame for several minutes after baking. The black sample was then obtained after about 10 min. This process is called ECC technique. The as-grown black wool-like powder was characterized by JEOL 6500F high-resolution field-emission scanning electron microscopy (SEM), JEOL 2010 transmission electron microscopy(TEM), JEOL 2010F high-resolution field-emission transmission electron microscopy (HRTEM) and REINSHAW optical confocal Ramanspectroscopy.
3 Results and discussion
Massive products of solid-cored carbon nanowires were obtained using various catalysts by ECC technique. According to SEM investigation of as-grown carbon nanowires, it is found that the carbon nanowires are relatively thin with diameter less than 100 nm and a good uniformity when the catalyst precursor is iron nitrate (Fig.1(a)). And the carbon nanowires have a relatively large diameter commonly more than 100 nm with various types, such as helical structure, Y-shape structure when the catalyst precursor is nickel nitrate (Figs.1(c), (d)). When the catalyst precursor is cobalt nitrate, there are lots of defects to discriminate a complete nanowire (Fig.1(b)). It is obvious that the morphology of carbon nanowires is affected by catalyst precursor during ECC process.
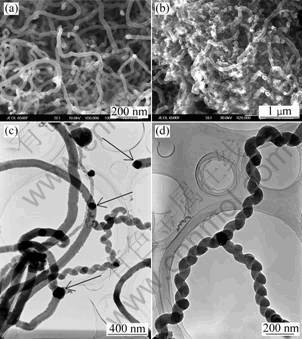
Fig.1 SEM and TEM images of carbon nanowires using catalyst precursor of iron nitrate (a), cobalt nitrate (b), nickel nitrate (c) and (d) (Arrows in Fig.1(c) mark catalysts)
The image of high-resolution TEM of carbon nanowires using iron nitrate as catalyst precursor shows that the nanowire is polycrystal consisting of nanocrystal of graphite. The white lines in Fig.2(a) mark the interface of the nanocrystals. The electron diffraction of carbon nanowires consists of concentric circles (Fig.2(b)), whose brightness is not uniform along a circle, indicating that the carbon nanowire is polycrystalline[10], in good agreement with HRTEM observation.
Raman spectra were obtained with a REINSHAW optical confocal Raman microscope and shown in Fig.3. The sample excitation was performed using 5 mW of 514.5 nm laser with 3 ?m spot size. All spectra show clear G peaks and D peaks, where D line in carbon materials is a defect-induced peak. The intensity of D peak is more stronger than that of G peak, which indicateds that the nanowires have a lot of defects or ultrafine graphite [11]. This is in good agreement with SEM and TEM observations.

Fig.2 HRTEM image of carbon nanowires (a) and electron diffraction graph of carbon nanowires (b)
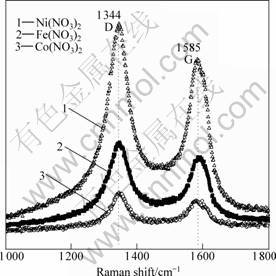
Fig.3 Raman spectra of carbon nanowires corresponding to different catalyst precursors
According to the experimental results, it can be known that different growth mechanisms correspond to different catalysts. TEM investigations show that the catalyst nanoparticles (see arrows in Fig.4(a)) locate at the end of carbon nanowires when the catalyst precursor is iron nitrate, while the catalyst nanoparticles (see arrows in Fig.1(c)) are at either the end or the middle of carbon nanowires when the catalyst precursor is nickel nitrate, and the catalysts nanoparticles (see arrows in Figs.4(b) and (c)) are at the middle of carbon nanowires when the catalyst precursor is cobalt nitrate.
Therefore, different catalysts lead to different growth mechanisms. It could be top-growth for iron nitrate precursor, top-growth or middle-growth for nickel nitrate precursor, and middle-growth for cobalt nitrate precursor.

Fig.4 TEM images of carbon nanowires with different catalyst precursors: (a) Iron nitrate; (b) and (c) Cobalt nitrate(Arrows mark catalysts)
For further analysis, the energy-dispersive X-ray spectra (EDS) were obtained(see Fig.5). The copper in EDS spectra comes from substrate. There is almost no oxygen in all EDS spectra. Therefore, metal oxide might be reduced by carbon species that were generated due to inadequate combustion of ethanol in inner flame.
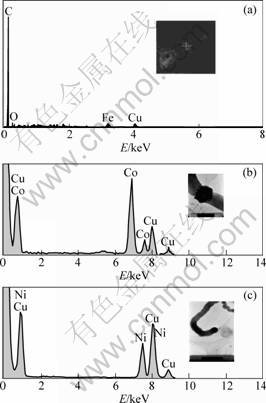
Fig.5 Energy dispersive X-ray spectra of catalysts from precursors: (a) Iron nitrate, white cross in inset marks catalyst; (b) Cobalt nitrate, dark dot at end of carbon nanowires in inset is catalyst; (c) Nickel nitrate, dark polygon in middle of carbon nanowires in inset is catalyst
The growth mechanism for helical structure is not clear. In the experiment it is also found the catalysts exist in the middle or at the end of nanowires. However, the helical structure could be associated with polycrystalline structure of carbon nanowires, although future work is required to better understand the effect of catalysts.
4 Conclusions
Carbon nanowires are synthesized by using ECC technique. The dependence of morphology, microstructure and growth mechanism of carbon nanowires on the catalyst is obtained. Iron nitrate or nickel nitrate will generate nanowires with relatively good uniformity, while cobalt nitrate precursor will generate nanowires with many defects. The relatively good carbon nanowires, particularly helical structure, could be used as electrode, reinforcement materials of polymer and other matrix systems, field emitter, and electro-magnetic absorbing materials.
References[1] ZHU H W, XU C L, WU D H, WEI B Q, VAJTAI R, AJAYAN P M, Direct synthesis of long single-walled carbon nanotube strands[J]. Science, 2000, 296: 884-886.
[2] NGUYEN P, HOU T N, MEGYAPPAN M. Catalyst metal selection for synthesis of inorganic nanowires[J]. Adv Mater, 2005, 17: 1773-1777.
[3] REN Z F, HUANG Z P, XU J W, WANG J H, BUSH P, SIEGAL M P, PROVENCIO P N. Synthesis of large arrays of well-aligned carbon nanotubes on glass[J]. Science, 1998, 282: 1105-1107.
[4] FAN Shou-shan, CHAPLINE M G, FRANKLIN N R, TOMBLER T W, CASSELL A M, DAI Hong-Jie. Self-oriented regular arrays of carbon nanotubes and their field emission properties[J]. Science, 1999, 283: 512-514.
[5] ZOU X P, ABE H, SHIMIZU T, ANDO A, NAKAYAMA Y, TOKUMOTO H, ZHU S M, ZHOU H S. Simple thermal chemical vapor deposition synthesis and electrical property of multi-walled carbon nanotubes[J]. Physica E, 2004, 24: 14-18.
[6] ZHENG L X, O’CONNELL M J, DOORN S K, LIAO X Z, ZHAO Y H, AKHADOV E A, HOFFBAUER M A, ROOP B J, JIA Q X, DYE R C, PETERSON D E, HUANG S M, LIU J, ZHU Y T. Ultralong single-wall carbon nanotubes[J]. Nature Materials, 2004, 3: 673-676.
[7] PAN Z W, XIE S S, CHANG B H, WANG C Y, LU L, LIU W, ZHOU W Y, LI W Z, QIAN L X. Very long carbon nanotubes[J]. Nature, 1998, 394: 631-632.
[8] WANG Y, SERRANO S, SANTIAGO-AVILES J J. Raman characterization of carbon nanofibers prepared using electrospinning[J]. Synthetic Metals, 2003, 138: 423-427.
[9] LEE C J, LEE T J, Park J. Carbon nanofibers grown on sodalime glass at 500 ℃ using thermal chemical vapor deposition[J]. Chem Phys Lett, 2001, 340: 413-418.
[10] TERRONES M, GROBERT N, OLIVARES J, ZHANG J P, TERRONES H, KORDATOS K, HSU W K, HARE J P, TOWNSEND P D, PRASSIDES K, CHEETHAM A K, KROTO H W, WALTON D R M. Controlled production of aligned-nanotube bundles[J]. Nature, 1997, 388: 52-55.
[11] SUN L F, LIU Z Q, MA X C, ZHONG Z Y, TANG S B, XIONG Z T, TANG D S, ZHOU W Y, ZOU X P, LI Y B, TAN K L, XIE S S, LIN J Y. Growth of carbon nanotube arrays using the existing array as a substrate and their Raman characterization[J]. Chem Phys Lett, 2001, 340: 222-226.
Foundation item: Project (KM200510772013) supported by the Science and Technology Development Program of Education Committee of Beijing City; Project(2005-2007) supported by Academic Innovative Team Program (Novel Sensor and Materials: Nanodevice and Nanomaterials) of Education Committee of Beijing City
Corresponding author: ZOU Xiao-ping; Tel: +86-10-64884673-812; Fax: +86-10-64879486; E-mail: xpzou2005@gmail.com


