Trans. Nonferrous Met. Soc. China 26(2016) 3299-3304
Boron removal from molten silicon using CaO-SiO2-BaO-CaF2 slag
Jin-ling SUN1, Jin-chuan JIE1, Qing-chuan ZOU1, Li-juan GUO2, Zhi-qiang CAO1, Tong-min WANG1, Ting-ju LI1
1. School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China;
2. TDK Dalian Corporation, Dalian 116024, China
Received 11 October 2015; accepted 19 September 2016
Abstract:
The distribution coefficient (LB) of boron between CaO-SiO2-BaO-CaF2 slag and silicon was investigated using electromagnetic induction melting for the purpose of improving the boron removal fraction. The dependence of the boron distribution coefficient between slag and silicon on the fundamental parameters of CaO to SiO2 mass ratio and refining time and the additions of BaO and CaF2 to the slag was discussed. The results show that LB can be increased by adding BaO and CaF2 to CaO-SiO2 slag. The maximum value of LB (6.94) is obtained when the CaO to SiO2 mass ratio is 1.1:1 and the contents of BaO and CaF2 are fixed at 15% and 20%, respectively. Increasing the refining time increases the LB. After the slag treatment is performed twice, the boron content of the silicon is successfully reduced from 3.5×10-5 to 3.7×10-6, and the removal fraction of boron reaches 89.4%.
Key words:
metallurgical silicon; boron removal; CaO-SiO2-BaO-CaF2;
1 Introduction
Because of environmental pollution and the depletion of fossil fuels, solar energy is an option to provide renewable energy to adapt to the demands of sustainable development. Silicon has been used as a starting material in the photovoltaic industry. In recent years, metallurgical grade silicon (MG-Si) [1] has been used as a feedstock alternative to substitute electronic grade silicon to solve the problem of silicon feedstock shortage. The purity of metallurgical grade silicon is 2N. Therefore, to satisfy the purity requirements of solar grade silicon (SOG-Si), it is necessary to remove a variety of impurities, such as phosphorus and boron impurities.
Theses metallic impurities can be removed by directional solidification because of their low segregation coefficient. Phosphorus can be removed using vacuum refining because of its high vapor pressure. However, boron is difficult to remove using either directional solidification [2,3] or vacuum treatment [4,5] because of its high segregation coefficient [6] and low vapor pressure. Slag treatment is an effective method to remove boron from metallurgical silicon. Boron reacts with slag to form boron oxides that transfer to the slag phase, whereas, refined silicon can easily be separated from the slag phase.
In recent years, various CaO-SiO2-based slag systems have been investigated to evaluate the removal of boron from metallurgical silicon [7-10]. TEIXEIRA et al [11,12] conducted systematic research to understand the thermodynamics of the CaO-SiO2 slag system. JOHNSTON et al [13,14] discussed the effects of slag basicity, oxygen potential and the mass ratio of slag to silicon on the boron removal process. LUO et al [15] studied the kinetics of boron removal using an electromagnetic induction melting process.
Apparently, the boron removal fraction is strongly dependent on slag chemistry. BaO is one of the most basic fluxes, which is preferred to remove acidic impurities such as boron. Besides, the slag basicity of BaO is stronger than that of CaO, and BaO can decrease the melting point and viscosity of the slag [16,17].
CaF2 can further reduce the viscosity and liquefaction temperature of the slag [18,19], promoting the mass transfer process. Therefore, BaO and CaF2 were chosen in the present study to improve the boron removal fraction.
The extraction of boron from silicon using CaO-SiO2-BaO-CaF2 slag has not been systematically studied. SUZUKI et al [8] only investigated the effect of the CaO to SiO2 mass ratio on extraction using CaO-SiO2-BaO-CaF2 slag. Therefore, in the present study, comprehensive data on the fundamental parameters including CaO to SiO2 mass ratio, refining time, additions of BaO and CaF2 to the slag and repeated slag treatment have been investigated. The values of the boron distribution coefficient between slag and silicon (LB) of CaO-SiO2-15%BaO-20%CaF2 and CaO-SiO2 were compared.
2 Experimental
CaO-SiO2-BaO-CaF2 slag was used to remove the boron. Although liquid slag is an ionic melt composed of cations and anions, the F- ions in the slag are assumed to exist with Ca2+, and this component is expressed as CaF2. All of the oxides and CaF2 were reagent grade. The metallurgical grade silicon that was used as the raw material in this study was supplied by Xinlong Co., Ltd. The boron content of this MG-Si was 3.5×10-5. The effects of varying the CaO to SiO2 mass ratio, refining time, as well as the BaO and CaF2 proportions were studied to obtain high values of LB. The CaO to SiO2 mass ratio was varied from 0.3:1 to 1.1:1 with the BaO and CaF2 contents fixed at 5% and 10%, respectively. The refining time varied from 1200 to 6000 s. BaO content varied from 0 to 35%, and CaF2 content varied from 0 to 40%. The LB values of CaO-SiO2-15%BaO- 20%CaF2 slag and CaO-SiO2 slag were also compared.
The experimental parameters are shown in Table 1. The experimental process consisted of the following steps. The metallurgical silicon was ground to a particle size of 0.1-0.2 mm. The metallurgical grade silicon was then washed with acetone in an ultra-sonic cleaner to remove possible solid residue from the surface. After drying, 0.3 kg of metallurgical silicon and 0.075 kg of slag (silicon to slag mass ratio of 4:1) were placed in a pure graphite cylindrical crucible (inner diameter: 0.06 m; outer diameter: 0.07 m; height: 0.16 m) that was surrounded by a graphite holder (inner diameter: 0.07 m; outer diameter: 0.09 m; depth: 0.16 m). The slag layer was placed in the middle of the graphite crucible. Then, the crucible was loaded into an intermediate frequency (2000 Hz) induction melting furnace. The schematic diagram of the apparatus is provided in Fig. 1. The basic equipment consisted of a rotary vane mechanical vacuum pump, a diffusion vacuum pump, a feeding device, an induction coil heating system and an argon gas inlet. Firstly, the rotary vane mechanical vacuum pump and the diffusion vacuum pump evacuated the furnace. When the absolute pressure was below 30 Pa, electric power was applied to melting the metallurgical silicon and slag, and argon gas was added to the furnace to maintain a pressure of approximately 1.0×104 Pa. An infrared thermometer was used to measure the temperature. Two colors of pyrometer were used in the present experiments and the wavelength is 1.0 μm. The smelting continued for 1200-6000 s at 1873 K under the argon atmosphere. After refining, the silicon and slag were physically separated, and the boron contents of the silicon and the slag were analyzed by inductively coupled plasma atomic emission spectrometry (ICP-AES).
Table 1 Experimental parameters used in this study
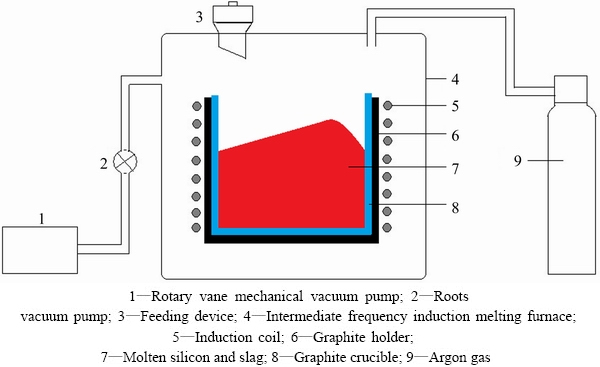
Fig. 1 Schematic drawing of intermediate frequency induction furnace
The distribution coefficient of boron between the slag and silicon phase was defined as follows:
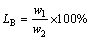 (1)
(1)
where w1 and w2 are the contents of boron in slag and silicon, respectively.
3 Results and discussion
Figure 2 shows the longitudinal section of the silicon ingot. The height of the ingot was 0.054 m and the radius of the ingot was 0.06 m. It is clearly seen that most of the slag is distributed at the top of the silicon. The slag can be effectively separated from silicon in the present study.

Fig. 2 Longitudinal section of silicon ingot
3.1 Effect of refining time on LB
The refining time is an important parameter influencing the LB value. The effect of refining time on LB was investigated over the range of 1200-6000 s, and the CaO to SiO2 mass ratio and the BaO and CaF2 contents were fixed at 1.1:1, 5% and 10%, respectively. Figure 3 shows the relationship between refining time and LB, indicating that LB increased as the refining time increased. The boron content was reduced from 3.5×10-5 in the raw MG-Si to 1.6×10-5 at 6000 s, and the LB increased to 4.75. Most of the boron was eliminated during the first 3600 s, and the slope of the curve became moderate after 4800 s, indicating that further increasing the refining time cannot contribute much to the removal fraction. From this result, 3600 s was selected to be optimal for refining the molten silicon. This may be attributed to the forced melt circulation caused by the electromagnetic force.
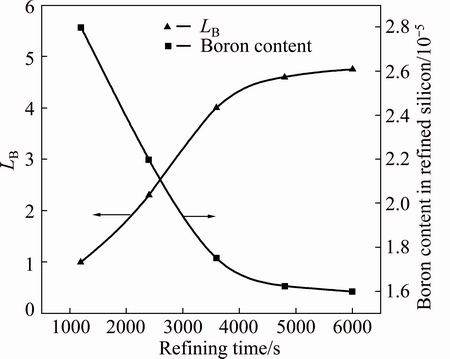
Fig. 3 Effect of refining time on boron removal
3.2 Effect of CaO to SiO2 mass ratio on boron removal
Figure 4 shows the relationship between CaO to SiO2 mass ratio and LB. The mass ratio of CaO to SiO2 varied from 0.3:1 to 1.1:1, and, the amounts of BaO and CaF2 were fixed at 5% and 10%, respectively. The mass ratio of silicon to slag was 4:1, and the melt was held at 1873 K for 3600 s. The LB decreased first and then increased with the increasing CaO to SiO2 mass ratio. The minimum LB (1.89) was obtained at the CaO to SiO2 mass ratio of 0.7:1. The maximum LB (4) was obtained at the CaO to SiO2 mass ratio of 1.1:1. These results are similar to those published by TEIXEIRA et al [11].
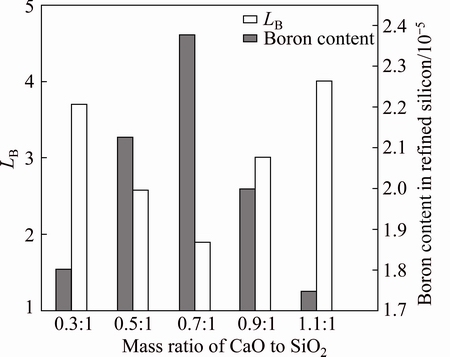
Fig. 4 Effect of CaO to SiO2 mass ratio on boron removal
When the CaO to SiO2 mass ratio was less than 0.7:1, the decrease of the CaO to SiO2 mass ratio resulted in an increase of the amount of SiO2 and oxygen partial pressure, contributing to an increase of LB. When the CaO to SiO2 mass ratio was above 0.7:1, the increase of the CaO to SiO2 mass ratio resulted in a decrease of the viscosity as well as the oxygen ion of slag, which was attributed to the decomposition of the silicon-oxygen complex ions. Thus, LB increased with increasing CaO to SiO2 mass ratio when the CaO to SiO2 mass ratio was above 0.7:1.
Figure 5 compares the results of the present study with those of other researchers. Our curve is similar to the work of ZHANG et al [20] and TEIXEIRA et al [11] with CaO-SiO2 slag and differs from the work of CAI et al [7]. The LB value found in the present study is somewhat lower than that determined by TEIXEIRA et al [11] and CAI et al [7]. In the work of TEIXEIRA et al [11] with CaO-SiO2 slag, the refining time was 64800 s and the slag attained the equilibrium and in our research the slag did not reach equilibrium. In the work of CAI et al [7], the mass ratio of slag to silicon was 3:1, contributing to the higher LB. The LB value determined in the present study was also lower than that determined by ZHANG et al [20], because of the lower BaO and CaF2 contents. When the BaO and CaF2 contents were 15% and 20%, respectively, the LB value determined in the present study reached 6.94. The LB values in the work of TEIXEIRA et al [11] with CaO-SiO2-25%CaF2 slag and CaO-SiO2-40%CaF2 are lower than those of others, which may be attributed to the higher CaF2 content. When the CaF2 content is too high, it contributes to the dilution of the slag. According to the study of WU et al [10], the LB of CaO-SiO2 slag is lower than that determined in our study. The addition of 5% BaO and 10% CaF2 may contribute to the higher LB values of our results.
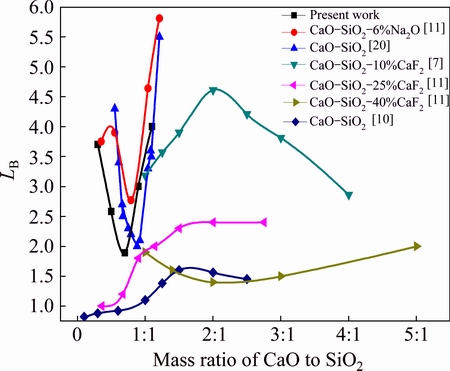
Fig. 5 Comparing results of present study with those of other researchers [7,10-12,20]
3.3 Effect of BaO content on LB
The influence of BaO addition to the slag on LB was investigated at 1873 K for 3600 s, and the results are shown in Fig. 6. The BaO contents were 0, 5%, 15%, 25% and 35%, and the CaO to SiO2 mass ratio and CaF2 content were fixed at 1.1:1 and 10%, respectively. The LB initially increased with BaO addition to the slag, reaching a maximum value of 6.18 when the BaO content was 15%. Further BaO addition to the slag resulted in decreasing LB.
Addition of small amounts of BaO may decrease the melting point and viscosity of the slag [16,17], resulting in an increase of LB. However, a large amount of BaO may also increase the melting point and viscosity of the slag. If the viscosity of the slag increases significantly with a large amount of BaO, the value of LB will decrease in a limited refining time.
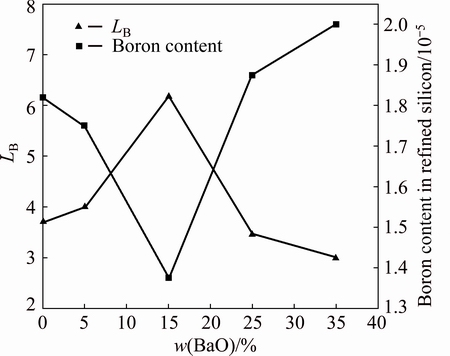
Fig. 6 Effect of BaO content on boron removal
3.4 Effect of CaF2 content on LB
The influence of CaF2 addition to the slag on LB was investigated by varying the content of CaF2, and the results are presented in Fig. 7. The CaO to SiO2 mass ratio and BaO content were fixed at 1.1:1 and 15%, respectively. The contents of CaF2 were 0, 10%, 20%, 30% and 40%. The LB increased and then decreased with increasing CaF2, reaching a maximum value of 6.94 when the CaF2 content was 20%.
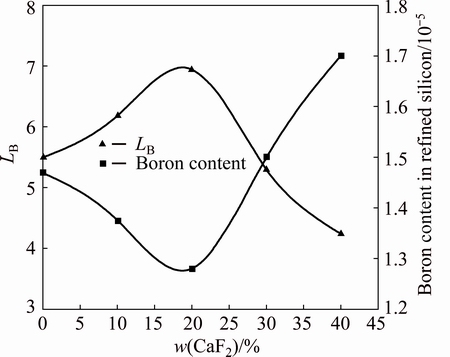
Fig. 7 Effect of CaF2 content on boron removal
A small addition of CaF2 may decrease the melting point and viscosity of the slag [18], resulting in an increase of LB. However, a larger amount of CaF2 may also increase the viscosity [21] with increasing the melting point of the slag, resulting in a decrease of the LB. In addition, when the CaF2 content was greater than 20%, the amount of the SiO2 decreased and CaF2 significantly diluted the slag, resulting in a decrease of LB.
3.5 Boron removal using repeated slag treatment
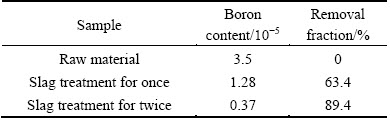
The maximum LB was obtained when the CaO, SiO2, BaO and CaF2 contents were 34.1%, 30.9%, 15% and 20%, respectively. Thus, 34.1%CaO-30.9%SiO2-15%BaO-20%CaF2 slag was chosen for repeated slag treatment. The smelting was conducted for 3600 s at 1873 K, and the mass ratio of silicon to slag was 4:1. The results presented in Table 2 show the boron content of the silicon following single and repeated slag treatment. After the initial slag treatment, the boron content of the silicon was reduced from 3.5×10-5 to 1.28×10-5, and the boron removal fraction was 63.4%. After the second slag treatment, the boron content of the silicon was reduced to 3.7×10-6, and the total boron removal fraction was 89.4%. Thus, boron can be effectively removed through repeated slag treatment.
Table 2 Boron content in silicon after slag treatment for once and twice
3.6 Comparing LB value of CaO-SiO2-15%BaO- 20%CaF2 and CaO-SiO2
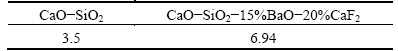
Table 3 compares the LB value of CaO-SiO2- 15%BaO-20%CaF2 with that of CaO-SiO2. The CaO to SiO2 mass ratio was fixed at 1.1:1, and the mass ratio of silicon to slag was 4:1, with 3600 s of refining at 1873 K. After CaO-SiO2-15%BaO-20%CaF2 slag refining, LB reached 6.94. After CaO-SiO2 slag refining, LB reached only 3.5. Table 3 shows that the addition of 15% BaO and 20% CaF2 increased the LB.
Table 3 Comparing LB values between CaO-SiO2-15%BaO- 20%CaF2 and CaO-SiO2
4 Conclusions
1) The LB increased with increasing the refining time. Most of the boron was eliminated during the first 3600 s, and 3600 s was selected to be optimal for refining the molten silicon.
2) The maximum LB value of 6.94 was obtained by using slag with CaO to SiO2 mass ratio of 1.1:1 and 15% BaO together with 20% CaF2.
3) After the second slag treatment, the boron content of the silicon was reduced to 3.7×10-6, and the total boron removal fraction was achieved up to 89.4%.
References
[1] NAKAMURA N, BABA H, SAKAGUCHI Y, KATO Y. Boron removal in molten silicon by a steam-added plasma melting method [J]. Materials Transactions, 2004, 45(3): 858-864.
[2] GANESH R B, RYNINGEN B, SYVERTSEN M, OVRELID E, SAHA I, TATHGAR H, RAJESWARAN G. Growth and characterization of multicrystalline silicon ingots by directional solidification for solar cell applications [J]. Energy Procedia, 2011, 8(4): 371-376.
[3] MARTORANO M A, NETO J B F, OLIVEIRA T S, TSUBAKI T O. Refining of metallurgical silicon by directional solidification [J]. Material Science and Engineering B, 2011, 176(3): 217-226.
[4] ZHENG Song-sheng, ENGH T A, TANGSTAD M, LUO Xuo-tao. Separation of phosphorus from silicon by induction vacuum refining [J]. Separation & Purification Technology, 2011, 82: 128-137.
[5] ZHENG Song-sheng, CHEN Wen-hui, CAI Jing, LI Jin-tang, CHEN Chao, LUO Xue-tao. Mass transfer of phosphorus in silicon melts under vacuum induction refining [J]. Metallurgical & Materials Transactions B, 2010, 41(6): 1268-1273.
[6] MORITA K, MIKI T. Thermodynamics of solar-grade-silicon refining [J]. Intermetallics, 2003, 11(11-12): 1111-1117.
[7] CAI Jing, LI Jin-tang, CHEN Wen-hui, CHEN Chao, LUO Xue-tao. Boron removal from metallurgical silicon using CaO-SiO2-CaF2 slags [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(6): 1402-1406.
[8] SUZUKI K, SUGIYAMA T, TAKANO K, SANO N. Thermodynamics for removal of boron from metallurgical silicon by flux treatment [J]. Journal of the Japan Institute of Metals, 1990, 54(2): 168-172.
[9] LI Yan-long, WU Ji-jun, MA Wen-hui. Kinetic of boron removal from metallurgical grade silicon using a slag refining technique based on CaO-SiO2 binary system [J]. Separation Science & Technology, 2014, 49(12): 1946-1952.
[10] WU Ji-jun, LI Yan-long, MA Wen-hui, WEI Kui-xian, YANG Bin, DAI Yong-nian. Boron removal in purifying metallurgical grade silicon by CaO-SiO2 slag refining [J]. Transactions of Nonferrous Metals Society of China, 2014, 24: 1231-1236.
[11] TEIXEIRA L A V, TOKUDA Y, YOKO T, MORITA K. Behavior and state of boron in CaO-SiO2 slags during refining of solar grade silicon [J]. Transactions of the Iron & Steel Institute of Japan, 2009, 49(6): 777-782.
[12] TEIXEIRA L A V, MORITA K. Removal of boron from molten silicon using CaO-SiO2 based slags [J]. ISIJ International, 2009, 49(6): 783-787.
[13] JOHNSTON M D, BARATI M. Effect of slag basicity and oxygen potential on the distribution of boron and phosphorus between slag and silicon [J]. Journal of Non-Crystalline Solids, 2011 357(3): 970-975.
[14] JOHNSTON M D, BARATI M. Distribution of impurity elements in slag-silicon equilibria for oxidative refining of metallurgical silicon for solar cell applications [J]. Solar Energy Materials & Solar Cells, 2010, 94(12): 2085-2090.
[15] LUO Da-wei, LIU Ning, LU Yi-ping, ZHANG Guo-liang, LI Ting-ju. Removal of boron from metallurgical grade silicon by electromagnetic induction slag melting [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(5): 1178-1184.
[16] LI Gui-rong. Effect of strong basic oxide (Li2O, Na2O, K2O and BaO) on property of CaO-based flux [J]. Journal of Iron & Steel Research. 2003, 10(3): 6-9.
[17] LU Yan-qing, FANG Xing, ZHANG Guo-dong. Effects of BaO on properties of low fluoride content mould flux for high speed continuous casting [C]//Proceedings of International Conference on Advanced Engineering Materials and Technology. Kunming: Advanced Materials Research , 2011: 1866-1870. (in Chinese)
[18] GAO Jin-xing, WEN Guang-hua, HUANG Ting, TANG Ping, LIU Qiang. Effect of the composition on the structure and viscosity of the CaO-SiO2 based mold flux [J]. Journal of Non-crystalline Solids. 2016, 435: 33-39.
[19] TAKEDA O, OKAWARA T, SATO Y. Development of a new viscometer based on rotating crucible method and viscosity measurement of SiO2-CaO-CaF2 system [J]. ISIJ International, 2012, 52(9): 1544-1549.
[20] ZHANG Lei, TAN Yi, LI Jia-yan, LIU Yao, WANG Deng-ke. Study of boron removal from molten silicon by slag refining under atmosphere [J]. Material Science in Semiconductor Processing, 2013, 16(6): 1645-1649.
[21] HEO J H, CHUNG Y, PARK J H. Effect of CaF2 addition on the silicothermic reduction of MnO in ferromanganese slag [J]. Metallurgical & Materials Transactions B, 2015, 46(3): 1154-1161.
CaO-SiO2-BaO-CaF2四元渣去除熔硅中的硼杂质
孙金玲1,接金川1,邹清川1,郭丽娟2,曹志强1,王同敏1,李廷举1
1. 大连理工大学 材料科学与工程学院,大连 116024;
2. TDK大连电子有限公司,大连 116024
摘 要:为提高硼的去除率,研究了电磁感应精炼过程中硼杂质在CaO-SiO2-BaO-CaF2四元渣和熔硅之间的分配系数LB,讨论了四元渣系中CaO/SiO2质量比、BaO和CaF2含量、熔炼时间对LB的影响规律。结果表明:随着CaO-SiO2渣中BaO和CaF2含量的增大,LB值增大。当CaO/SiO2质量比为1.1:1、BaO和CaF2含量分别为15%和20%时,CaO-SiO2-BaO-CaF2四元渣去除熔硅中硼杂质效果最好,LB达到最大值6.94,并且LB随着熔炼时间的延长而增大。经过两次造渣后,熔硅中硼含量由3.5×10-5降到3.7×10-6,硼的去除率达到89.4%。
关键词:冶金级硅;除硼;CaO-SiO2-BaO-CaF2
(Edited by Xiang-qun LI)
Foundation item: Projects (51501028, 51471042, 51375070) supported by the National Natural Science Foundation of China; Project supported by the Fundamental Research Funds of the Central Universities, China
Corresponding author: Jin-chuan JIE; Tel: +86-15941130325; E-mail: jiejc@dlut.edu.cn
DOI: 10.1016/S1003-6326(16)64464-3
Abstract: The distribution coefficient (LB) of boron between CaO-SiO2-BaO-CaF2 slag and silicon was investigated using electromagnetic induction melting for the purpose of improving the boron removal fraction. The dependence of the boron distribution coefficient between slag and silicon on the fundamental parameters of CaO to SiO2 mass ratio and refining time and the additions of BaO and CaF2 to the slag was discussed. The results show that LB can be increased by adding BaO and CaF2 to CaO-SiO2 slag. The maximum value of LB (6.94) is obtained when the CaO to SiO2 mass ratio is 1.1:1 and the contents of BaO and CaF2 are fixed at 15% and 20%, respectively. Increasing the refining time increases the LB. After the slag treatment is performed twice, the boron content of the silicon is successfully reduced from 3.5×10-5 to 3.7×10-6, and the removal fraction of boron reaches 89.4%.


