
Treatment of naphthalene derivatives with iron-carbon micro-electrolysis
WANG Yu-ping(王玉萍)1, 2, WANG Lian-jun(王连军)1,
PENG Pan-ying(彭盘英)2, LU Tian-hong(陆天虹)2
1. School of Chemical Engineering, Nanjing University of Science and Technology,
Nanjing 210094, China;
2. School of Chemistry and Environmental Science, Nanjing Normal University, Nanjing 210097, China
Received 26 March 2006; accepted 20 June 2006
Abstract:
The degradation of five naphthalene derivatives in the simulated wastewater was investigated using the iron-carbon micro-electrolysis method. The optimal initial pH of solution and adsorption of iron-carbon and removal efficiency of the total organic carbon(TOC) were investigated. The results show that the removal efficiency of the naphthalene derivatives can reach 48.9%-92.6% and the removal efficiency of TOC is 42.8%-78.0% for the simulated wastewater with 200 mg/L naphthalene derivatives at optimal pH of 2.0-2.5 after 120 min treatment. The degradation of five naphthalene derivatives with the micro-electrolysis shows the apparent first-order kinetics and the order of removal efficiency of the naphthalene derivatives is sodium 2-naphthalenesulfonate, 2-naphthol, 2, 7-dihydroxynaphthalene, 1-naphthamine, 1-naphthol-8-sulfonic acid in turn. It is illustrated that the substituents of the naphthalene ring can affect the removal efficiency of naphthalene due to their electron-withdrawing or electron-donating ability.
Key words:
cast iron scrap; micro-electrolysis; naphthalene; substituent; wastewater treatment;
1 IntroductionNaphthalene and its derivates are important industrial chemicals and are used extensively in dye and pharmacy industry. The wastewater contains naphthalene dyestuffs and its intermediates are characterized by darker color, higher concentration and toxicity, and is difficult to be degraded under natural environment[1]. Naphthalene ring has delocalization conjugated bond composed of ten carbon atoms and this structure is quite stable, so usual physical and chemical methods such as adsorption, flocculating settling and biodegradation have hardly satisfied effect[2,3]. Therefore it is very meaningful to look for the economical and practical technology that can be used for processing wastewater of naphthalene series.
Micro-electrolysis (also called iron chip filtration) has been one of the most effective pretreatment methods of the wastewater [4]. The iron and carbon in cast iron scrap (or added hard coke) can form a primary battery in the electrolyte solution. The products of electrode have
high activity, and can cause electro-coagulation and redox reaction with many components. Therefore organic compounds are decomposed and decolorized[5]. The micro-electrolysis as a pretreatment method of the wastewater has been reported to treat the wastewater in the dye-printing, electroplating and petrochemical industry[4]. This method can improve the biodegradability of the wastewater[6] because it can be extensively used in many kinds of wastewater and the treating cost is low. Researches of iron-carbon micro- electrolysis at present are focused on decolourization of wastewater of dye[7] and reduction of organics with iron [8,9]. But few papers are reported about the effect of the structure of the organic compounds on removal efficiency in the micro-electrolysis.
In this study, the removal efficiency of naphthalene derivatives using the iron-carbon micro-electrolysis method is studied. By comparing the removal efficiency of naphthalene derivatives with that of total organic carbon(TOC) and that by using FeSO4, the mechanism of removal of naphthalene derivatives using iron-carbon micro-electrolysis method is determined, and the effect of the substituents of the naphthalene derivatives on the removal efficiency is investigaed.
2 Experimental2.1 Materials
2-naphthol(99.5%)(NO), 1-naphthamine (99%) (NM), sodium 1-naphthalenesulfonat(99%)(NS), 2, 7- dihydroxynaphthalene(98.31%)(DHN) and 1-naphthol-8- sulfonic acid(98%)(NSA) were purchased from Pingyuan Zhangchen Chemical Co Ltd and used as received. The cast iron scrap was obtained from The Second Nanjing Machine Tool Work. The cast iron scrap was immersed firstly for 120 min in 5% NaOH solution to remove the oil film on the surface and then washed with water until the eluate was neutral. Before being used, it was immersed in the dilute sulfuric acid of pH=3 for 30 min to remove the oxidation film on the surface in order to increase the surface area and the activation. Then, it was washed with water until the eluate was neutral. The activated carbon with the particle size of 0.42-0.84 mm was washed with water and dried at 100 ℃. Before use, it was absorbed in the measured solution until the equilibrium was reached.
2.2 Experimental procedure
2.2.1 Iron-carbon micro-electrolysis method
The cast iron scrap and activated carbon were mixed with the mass ratio of iron to carbon of 5?1 in the organic glass reactor. 500 mL simulated wastewater with 200 mg/L naphthalene derivative was added into the reactor and the pH value of the mixture was adjusted with the dilute sulfuric acid. Then, the compressed air was pumped into the mixture to stir it and provide enough oxygen for the micro-electrolysis reaction. Sampling was carried out every 30 min. The pH value of the simulated wastewater with naphthalene derivatives was measured with ORION-818 acidometer (Orion Electrochemical Analytical Instrument Company, America). The concentration of the naphthalene derivatives in the upper clear solution was determined with Carry50 ultraviolet spectrophotometer (Varian, America). Then, removal efficiency(Eo) of the naphthalene derivative can be calculated by [(c0-ct)/ c0]×100%, where c0 is the initial concentration of naphthalene derivative, and ct is the concentration of naphthalene derivative at different reacting time. TOC analyzer (TOC-VCSN, Shimadzu) was used to measure removal efficiency(Ec) of TOC.
In order to study the effect of pH value on Eo, five 500 mL simulated wastewaters with 100 mg/L naphthalene derivative were adjusted to pH value of 1.5, 2.0, 2.5, 3.0 and 3.5, respectively, with dilute sulfuric acid. Then, the cast iron scrap and active carbon (mass ratio of 5?1) were added into the simulated wastewaters [10]. Eo at 120 min was determined.
2.2.2 Iron-carbon adsorption method
The iron-carbon adsorption method was used to measure the amount of the naphthalene derivative adsorbed on the iron-carbon mixture. Its procedure was the same as the above iron-carbon micro-electrolysis method except no adjusting the pH value of the solution. Ea is expressed as the removal efficiency of the naphthalene derivatives for the iron-carbon adsorption method.
3 Results and discussion3.1 Effect of pH of solution on Eo
Fig.1 shows the relationship between the pH of the simulated wastewater and Eo at 120 min for each naphthalene derivative. It is observed from curves 1 and 3 in Fig.1, the pH shows a large effect on Eo. The largest Eo is obtained at pH=2.0 for the simulated wastewater with NSA and pH=2.5 for the simulated wastewaters with NM. For DHN (Fig.1, curve 4), the pH shows the small effect on Eo and its largest Eo is located at pH=2.5. For NO and NS (Fig.1, curves 2 and 5), when the pH is less than 2.5, the pH has small effect on Eo. However, when the pH is larger than 2.5, Eo is significantly decreased with increasing the pH. The largest Eo is located at pH=2.5. In balance, the largest Eo is obtained at pH=2.0 for the simulated wastewater with 1-naphthol-8-sulfonic acid and pH=2.5 for the simulated wastewaters with other naphthalene derivatives.
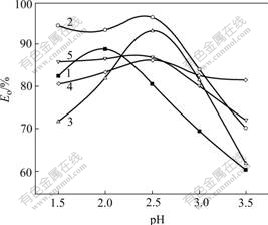
Fig.1 Relationship between Eo at 120 min and pH of simulated wastewater with different naphthalene derivatives: 1 NSA; 2 NO; 3 NM; 4 DHN; 4 NS
3.2 Comparison of Eo and Ea
Fig.2 shows the relationship between reaction time and removal efficiency for the simulated wastewater with the different naphthalene derivatives at the optimal pH.
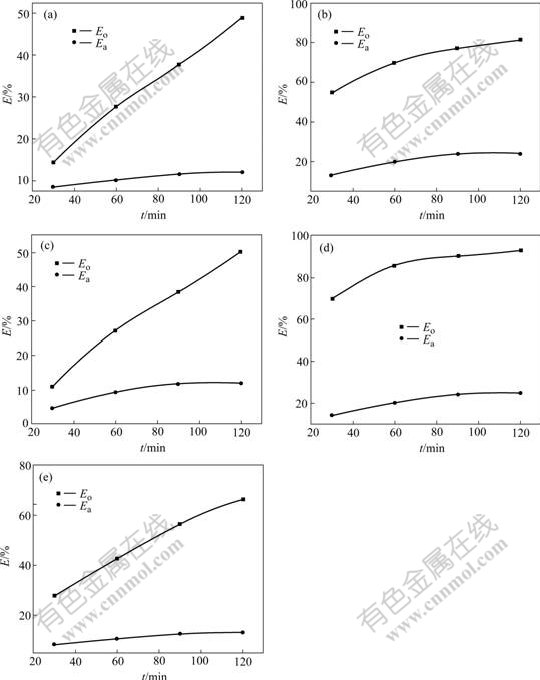
Fig.2 Relationship between reaction time with removal efficiency for simulated wastewater with different naphthalene derivatives at optimal pH: (a) NSA; (b) NO; (c) NM; (d) NS; (e) DHN
It is observed from Fig.2 that Eo is much larger than Ea for each naphthalene derivative. The results demonstrate that for removing the naphthalene derivatives in the wastewater, the micro-electrolysis method is much better than the absorption method because the naphthalene derivatives can be adsorbed on carbon in the cast iron scrap. But in the micro-electrolysis method, other physical and chemical actions would play an important role.
3.3 Eo and Ec of naphthalene derivatives
Fig.3 shows the relationship between the reaction time and removal efficiency for the simulated wastewaters with the different naphthalene derivatives at the optimal pH. It is found that the pH of the simulated wastewaters is increased with increasing the reaction time. After 120 min reaction, the pH of the simulated wastewaters changes from 2.0 or 2.5 to 4.7-4.9. It is observed from Fig.3 that after 120 min reaction, the order of Eo for the five naphthalene derivatives is NS(92.6%)>NO(81.3%)>DHN(66.0%)>NM(50.1%)>NSA(48.9%) and the order of Ec of five naphthalene compounds is as the same as that of EO, i.e. NS(78.0%)>NO(69.1%)>DHN (58.1%)>NM(43.7%)>NSA(42.8%). In addition, it is found that Eo is larger than Ec for each naphthalene derivative because Eo measurement is based on the concentration of the naphthalene derivative, but Ec measurement is based on the amount of the total organic carbon. It is illustrated that there are physical and chemical actions and intermediates in the iron-carbon micro electrolysis.
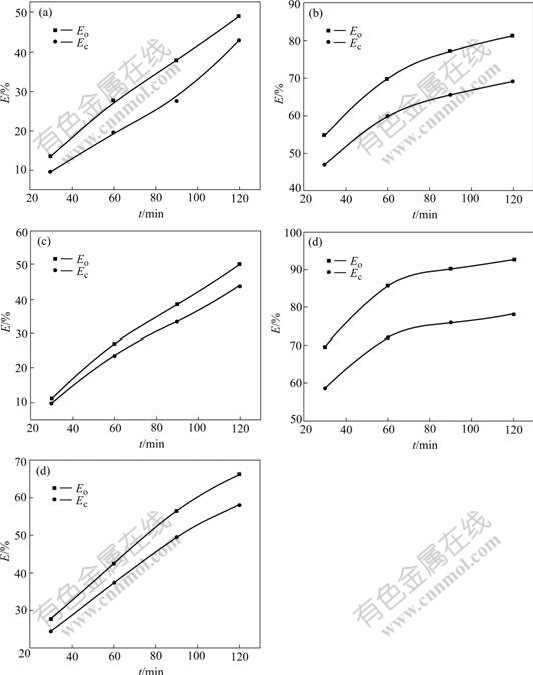
Fig.3 Relationship between reaction time and removal efficiency for simulated wastewaters with different naphthalene derivatives at optimal pH: (a) NSA; (b) NO; (c) NM; (d) NS; (e) DHN
3.4 Kinetics of degradation of naphthalene derivatives in micro-electrolysis
The relationship curves of ln(c0/ct) with the reaction time for the simulated wastewaters with different naphthalene derivatives at the optimal pH are shown in Fig.4.
It can be observed from Fig.4 that ln(c0/ct) for each naphthalene derivative almost linearly changes with the reaction time within the range of the experimental concentration. Therefore, the apparent first-order kinetic equation can be used:
ln(c0/ct)=kt (1)
where k is the apparent first order rate constant, min-1. k and half-lives(t1/2) for each naphthalene derivative can be calculated. The data are listed in Table 1. The linear correlation coefficients are 0.990-0.999.
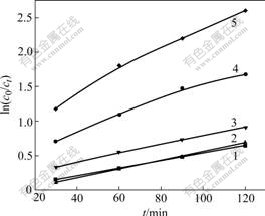
Fig.4 Relationship curves of ln(c0/ct) vs reaction time for simulated wastewater with different naphthalene derivatives at optimal pH: 1 NSA; 2 NO; 3 NM; 4 NS; 5 DHN
Table 1 k and t1/2 values for five naphthalene derivatives
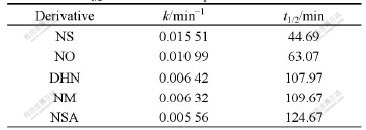
It can be found from Table 1 that the substituents can significantly affect the reactivity of the naphthalene derivatives in the micro-electrolysis. The order of k for the five naphthalene derivatives in the micro-electrolysis is NS>NO>DHN>NM>NSA. The result is in agreement with that of Eo.
3.5 Mechanism for effect of substituents on Eo of naphthalene derivatives
When FeSO4 is used to replace the cast iron scrap and the other procedures are the same as those in the micro-electrolysis, Eo values at 120 min for the different concentrations of FeSO4 and for the simulated wastewater with sodium 2-naphthalenesulfonate are listed in Table 2. The results in Table 2 illustrate that the action of FeSO4 is similar to the cast iron scrap. In addition, it is found that Eo at 120 min for the simulated wastewater with sodium 2-naphthalenesulfonate is increased with increasing the concentration of FeSO4. The only difference between the micro-electrolysis and FeSO4 treatment is that the pH does not change with the reaction time in the FeSO4 treatment.
Table 2 Effect of FeSO4 on removal efficiency of NS

It is reported that the cast iron scrap can form a primary micro battery with carbon in the electrolyte solution. The basic reactions in the micro battery are as follows[11]:
Anode reaction: Fe→Fe2++2e
Cathode reaction: 2H++2e→2[H]→H2
Total reaction: Fe+2H+→Fe2++H2
Thus, the nascent Fe2+ can be produced in the iron-carbon micro-electrolysis. It can be oxidized to Fe3+ with oxygen in the solution, forming Fe(H2O)63+, which can further form different hydroxyl irons, such as Fe(OH)2+ or ![]() . These hydroxyl irons have the strong flocculation[5]. They can form the floccules with the various organic compounds and then these organic compounds will precipitate from wastewater[12]. Then, the organic compound can be removed from the solution. It is obvious that when the pH of the solution is low, the reaction rate in the micro battery can be increased, leading to the increase in the concentration of Fe2+. Thus, Eo is increased. However, if the pH of the solution is too low, the hydroxyl irons are not easy to be formed so that Eo is decreased. Therefore, there is an optimal pH at 2.0 or 2.5 for the naphthalene derivatives. In addition, H+ is consumed in the micro battery reaction. Thus, during the treatment, the pH of the solution will be increased as indicated above.
. These hydroxyl irons have the strong flocculation[5]. They can form the floccules with the various organic compounds and then these organic compounds will precipitate from wastewater[12]. Then, the organic compound can be removed from the solution. It is obvious that when the pH of the solution is low, the reaction rate in the micro battery can be increased, leading to the increase in the concentration of Fe2+. Thus, Eo is increased. However, if the pH of the solution is too low, the hydroxyl irons are not easy to be formed so that Eo is decreased. Therefore, there is an optimal pH at 2.0 or 2.5 for the naphthalene derivatives. In addition, H+ is consumed in the micro battery reaction. Thus, during the treatment, the pH of the solution will be increased as indicated above.
The above results illustrate that the main removing mechanism of the naphthalene derivatives in the iron-carbon micro electrolysis is that the hydroxyl irons can form the floccules with the various organic compounds. Because the hydroxyl irons are the positive ions, they are easy to form the floccule with the compounds under the negative charge or high electron cloud density. Thus, the influence of the substituents on Eo of the naphthalene derivatives can be explained with the Hammett constant(σ)[13]. The Hammett constant represents the electron cloud density over a group[14]. A positive value of σ of a substitute indicates that the substitute is an electron-withdrawing group and a negative value represents an electron-donating group. For example, σ values of —NH2, —OH and —SO3H are -0.66, -0.37[13] and 0.50[15], respectively. MORAO[15] reported that a negative ion as a substituent possesses the electron donating ability and can increase the electron cloud density of the ring. Thus, a naphthalene derivative with a negative ion as a substituent is easy to interact with the hydroxyl iron ions. On the contrary, a positive ion as a substituent has the electron withdrawing ability and the electron cloud density of the naphthalene ring is decreased. Therefore, a naphthalene derivative with a positive ion as a substituent is difficult to interact with the hydroxyl iron ions. For NS and NM, the substituents of the naphthalene ring are —![]() and —
and —![]() , respectively. Thus, the hydroxyl iron ions are much easier to form the floccules with NS than with NM. There are two —OH groups in DHN, but only one in NO. The —OH group is an electron-donating group. However, it was reported [16,17] that the electro-donating ability of two —OH groups on the same naphthalene ring is less than that of one —OH group. Therefore, Eo of NO is higher than that of DHN. For NSA, there are one electron withdrawing group —SO3H and one electron-donating group —OH. —SO3H is adjacent to —OH. Perhaps, in the DHN, the interaction between —SO3H and —OH groups will decrease the electron cloud density largely. Thus, Eo of NSA is remarkably decreased.
, respectively. Thus, the hydroxyl iron ions are much easier to form the floccules with NS than with NM. There are two —OH groups in DHN, but only one in NO. The —OH group is an electron-donating group. However, it was reported [16,17] that the electro-donating ability of two —OH groups on the same naphthalene ring is less than that of one —OH group. Therefore, Eo of NO is higher than that of DHN. For NSA, there are one electron withdrawing group —SO3H and one electron-donating group —OH. —SO3H is adjacent to —OH. Perhaps, in the DHN, the interaction between —SO3H and —OH groups will decrease the electron cloud density largely. Thus, Eo of NSA is remarkably decreased.
4 Conclusions
1) The iron-carbon micro-electrolysis method can effectively remove the naphthalene derivatives from the simulated wastewater.
2) It is found that in the iron-carbon micro- electrolysis, the degradation of all of five naphthalene derivatives shows the apparent first-order kinetics and the order of Eo is NS>NO>DHN>NM>NSA.
3) Eo is related to the electron-donating ability or electron-withdrawing ability of substitents.
4) The main mechanism for removing the naphthalene derivatives is that the naphthalene derivatives can form the floccule with the hydroxyl iron ions produced from the reaction of the iron-carbon micro electrolysis.
References[1] XU Z Y, ZHANG Q X, CHEN J L, WANG L S, YANG G. Inhibition to activated sludge by naphthalene derivatives [J]. Environmental Chemistry, 1999, 18(6): 538-542. (in Chinese)
[2] FU C. Developments of treatment technology of naphthalene dye intermediate wastewater in China [J]. Dyestuff Industry, 2002, 25: 35-38. (in Chinese)
[3] FENG W G, . ZHANG Q X, CHEN J L. Review on the treatment processes for wastewater from the production of naphthalens series dye intermediate [J]. Environmental Protection of Chemical Industry, 1999, 19(4): 208-212. (in Chinese)
[4] ZHOU G P, FU D F. Application and development for micro-electrolysis technology [J]. Techniques and Equipment for Environmental Pollution Control, 2001, 2(4): 18-24. (in Chinese)
[5] ZHANG C Y. Research on Wastewater Treatment Techniques by Ferric-Carbon Micro-Electrolysis [D]. Nanjing: Southeast University, 2004. (in Chinese)
[6] HAO R X, CHEN S Y, HUANG Q X. Pretreatment of less-bio degradable printing and dyeing waste water by iron chipping- filtration process [J]. Environmental Protection of Chemical Industry, 1999, 19(3): 135-139. (in Chinese)
[7] JIN Y Z, ZHANG Y F, LI W. Micro-electrolysis technology for industrial wastewater treatment [J]. J of Environmental Sciences, 2003, 15(3): 334-338.
[8] LI Z, WILLIAN A A, RAYMOND M H. Kinetics of haloacetic acid reactions with Fe(0) [J]. Environ Sci Technol, 2004, 38: 6881-6889.
[9] MAREK S O, LAI G, ROBERT W G. Reduction of N-nitrosodimethylamine with granlar iron and nickel-enhanced iron: Mechanistic studies [J]. Environ Sci Technol, 2000, 34: 3495-3500.
[10] YUAN J X, WANG Y P, LIU Y, PENG P Y. The study on the treatment of 1-Naphthol-8-sulfonic simulated wastewater by iron- carbon micro-electrolysis technology [J]. Journal of Nanjing Normal University (Engineering and Technology), 2005, 27(1): 63-67. (in Chinese)
[11] XIAO X Y, CHEN Z H, CHEN Y C, LIU M Y. Papermaking middle-stage effluent treatment with micro-electrolysis method and its mechanism [J]. China Pulp and Paper, 2005, 24(7): 14-17. (in Chinese)
[12] FAN J H, XU W Y, GAO Y T. Mechanism and prospect of inner electrolysis technology in treating nitro aromatic compounds [J]. Environmental Protection Science, 2004, 30(123): 9-12. (in Chinese)
[13] PARRA S, OLIVERO J, PACHECO L, PULGARIN C. Structural properties and photo reactivity relationships of substituted phenols in TiO2 suspensions [J]. Applied Catalysis B: Environmental, 2003, 43: 293-301.
[14] LI S W, FAN R L. The Applied Handbook of Organic Chemistry [M]. Shanghai: Shanghai Science and Technology Press, 1981. 119-121. (in Chinese)
[15] MORAO I, HILLIER I H. Magnetic analysis(NICS) of monoarylic cations, linear relationship between aromaticty and Hammett constants(σp+) [J]. Tetrahedron Letter, 2001, 42: 4429-4431.
[16] LI X Y, LU B, MEN G P, ZHENG S J. HeI photoelectron spectroscopy studies and theoretical studied of aminonaphthalene and naphthol [J]. Journal of Hebei Normal University (Natural science), 2003, 27(6): 595-598. (in Chinese)
[17] McCULLOCH I, BAILEY C, GILE S M, HEENEY M, LOVE I, SHKUNOV M, SPARROWE D, TIERNEY S. Influence of molecular design on the field-effect transistor [J]. Chem Mater, 2005, 17(6): 1381-1385.
Foundation item: Project(05KJD6010110) supported by the Natural Science Foundation of the Education Commission of Jiangsu Province, China; Project(2005005) supported by the Science and Technology Foundation of the Environmental Protection Bureau of Jiangsu Province, China
Corresponding author: WANG Lian-jun; Tel: +86-25-84315518; E-mail: wanglj@mail.njust.edu.cn


