
Influence of NaCl deposition on atmospheric corrosion behavior of AZ91 magnesium alloy
ZHOU Wan-qiu(周婉秋)1, 2, SHAN Da-yong(单大勇)2, HAN En-hou(韩恩厚)2, KE Wei(柯 伟)2
1. College of Chemistry and Life Science, Shenyang Normal University, Shenyang 110034, China;
2. State Key Laboratory for Corrosion and Protection, Institute of Metal Research,Chinese Academy of Sciences, Shenyang 110016, China
Received 23 September 2009; accepted 30 January 2010
Abstract:
Corrosion behavior of AZ91 magnesium alloy under NaCl particle deposition condition was investigated by gravimetric method and surface analysis technique. It was found that the mass gain increased rapidly at the beginning of exposure and then slowly with time. The corrosion morphologies were observed and the results showed that NaCl deposition resulted in the occurrence of localized corrosion. The composition of corrosion product was analyzed using X-ray photo electron spectroscopy. It was suggested that the corrosion product was a mixture of oxide and hydroxide of magnesium and aluminum.
Key words:
magnesium alloy; atmospheric corrosion; NaCl deposition; X-ray photo electron spectroscopy;
1 Introduction
As the lightest structural metal material, magnesium alloys have great potential in automotive industry, aerospace industry and other industries where weight is of importance[1-2]. However, the use of magnesium alloys in engineering applications is mainly limited by their poor corrosion properties[3-4]. Despite the fact that magnesium alloys as structural materials are widely used in atmospheric environments, literature on atmospheric corrosion of magnesium alloys is scarce. Most of the studies focus on the electrochemical approach and the effect of microstructure in solutions[5-9]. The corrosion behavior in the atmosphere differs considerably from exposure in solution. It was indicated that the main cathodic process in solution is water reduction, while oxygen reduction is the main cathodic reaction during atmospheric corrosion with thin electrolyte layers, and the anodic process under thin electrolyte layers is diminished compared with a bulk electrolyte[10-11].
In the atmospheric environments, the corrosion rate was strongly influenced by the amount of NaCl deposited on the metal surface and it was accelerated when the relative humidity was increased[12-14]. Some field exposure researches indicated that corrosion products mainly consisted of magnesium carbonate. Hydromagnesite (Mg5(CO3)4(OH)2?4H2O) and Nesquehonite (MgCO3·3H2O) were the main corrosion products identified, and the corrosion form was uniform because of the existence of CO2 in the atmosphere[15]. The complexity of field exposure caused difficulty for realizing the influence of single factor on the corrosion product formation during the NaCl-induced atmospheric corrosion process. In this work, the corrosion behavior of AZ91 magnesium alloy was investigated with particular emphasis on corrosion rate, corrosion product composition under laboratory exposure conditions.
2 Experimental
2.1 Sample preparation
Cast AZ91D magnesium alloy was used as experimental material, and the composition of the alloy is given in Table 1. The samples had a superficial area of approximately 14.5 cm2 (with a dimension of 25 mm×20 mm×5 mm) and were sequentially polished to 2 000 grit. The samples were then degreased in acetone, dried in air, and stored in dry conditions.
The surface of magnesium alloy AZ91D was contaminated by different amounts of NaCl using a NaCl saturated solution containing 9% water+91% absolute
Table 1 Chemical composition of AZ91D magnesium alloy (mass fraction, %)
![]()
ethyl alcohol (volume fraction). Care was taken to contaminate equally the whole surface. The amount of NaCl was determined gravimetrically after being stored for 24 h in dry conditions. Three replicated samples were used for each exposure condition. The samples were placed in a box where the humidity was controlled by glycerin solution to achieve 90% relative humidity (RH). The samples were suspended vertically using a holder and exposed for 1 week, 2 weeks, 3 weeks, 4 weeks and 5 weeks, respectively. The samples were weighted before and after exposure.
2.2 Method of surface analysis
The morphologies of specimen were observed with an optical microscope. X-ray photo electron spectroscopy (XPS) measurements were performed in ESCALAB250 surface analysis system to analyse the composition of corrosion product.
3 Results and discussion
3.1 Corrosion induced by NaCl deposition
Fig.1 shows the mass gain of AZ91D as a function of exposure time at 90% RH and 25 ?C. The amounts of NaCl deposition were 50 and 120 μg/cm2 respectively. The mass gain increased rapidly after exposure for a week in humid condition, then slowed down with exposure time and was independent of the amount of NaCl. The corrosion rate of 120 μg/cm2 NaCl deposition was higher than that of 50 μg/cm2 NaCl deposition.
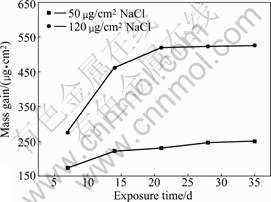
Fig.1 Relationship between mass gain and exposure time
In humid atmospheric environment, the NaCl grains absorbed the vapor in the air and formed thin electrolyte layers on the surface of metal. It was noted that droplets of NaCl solution were clearly visible on the surface, which led to the electrochemical corrosion under the thin electrolyte layers, and the mass gain was due to the formation of corrosion product. The corrosion rate decreased with exposure time, which implied that the initial corrosion product impeded the passage of corrosion medium and provided protection for metal substrate.
Fig.2 presents the surface images of samples after 3 weeks exposure. Corrosion occurred only on some locations and most of the metal surface were not attacked, which was in accordance with the work of LINDSTROM et al[10]. Many dispersed pits were seen on AZ91D and the morphologies showed typical characteristic of localized corrosion. The amount of NaCl deposition did not influence evidently the corrosion modality, however, more corrosion product was observed in high NaCl amount metal than that in low NaCl amount metal.
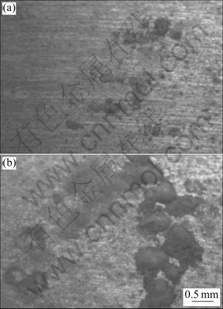
Fig.2 Corrosion morphologies of AZ91D induced by different amounts of NaCl deposition after exposure in humid air for 3 weeks: (a) 50 μg/cm2; (b) 120 μg/cm2
3.2 XPS analysis of corrosion product
XPS measurements were performed to obtain the chemical composition and structure of corrosion product. The specimens were sputtered by argon sputtering in vacuum chamber and XPS spectra were subsequently obtained. Fig.3 shows the XPS spectrum of the specimen after 3 weeks exposure under 50 μg/cm2 NaCl deposition in 90% RH for different sputtering time. Magnesium, aluminum, oxygen, chloride, sodium and carbon were found on the specimen surface, and the contaminated carbon diminished after initial sputtering.

Fig.3 XPS spectrum of corrosion product with different sputtering time
A stronger Mg peak appeared at 1 303.5 eV corresponding to Mg 1s. Al peak was relatively weak and the Al 2s signal could be detected at binding energy near 120.8 eV which boosted up with sputtering. A strong peak at 531.2 eV corresponding to O 1s reduced with sputtering time. At the binding energy of 198.2, 200.4 and 270.8 eV, very feeble chloride signal could be found which is corresponding respectively to Cl 2p1, Cl 2p3 and Cl 2s, and the chloride could not be further discovered after 600 s sputtering. The element sodium presented a relatively strong signal in the surface layer, Na KL1 and Na 1s were observed at 498.3 eV and 1 073.3 eV. The intensity of Na decreased with analysis depth, and Na peaks were very small at the layer which was 180 nm apart from the top surface.
The relationship between the molar fraction of elements and sputtering time in corrosion product is given in Fig.4. A relatively high oxygen content of 68% (molar fraction) was in the original sample surface, which sustained at about 50% for a long sputtering time. The oxygen content dropped gradually because of the little oxidation in inner layer.
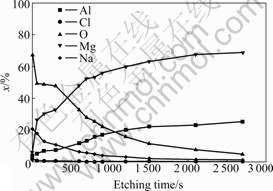
Fig.4 Effect of etching time on element contents in corrosion product
Magnesium was the main element in corrosion product, and its content increased with sputtering time. Aluminum content in corrosion product was about 10%-20% (molar fraction) which was much higher than that in AZ91 substrate, which implied that Al enrichment took place in the corrosion process. Fig.4 indicates that the sodium and chloride existed in corrosion product at a very low level and could only be found on the surface layer.
Fig.5 shows the XPS spectrum of Mg 1s in corrosion product. The peak appeared at binding energy of 1 303.7 eV which corresponded to magnesium oxide or hydroxide. The XPS spectrum of Al 2p characterized at binding energy of 74.4 eV was attributed to oxide or hydroxide of aluminum presented in Fig.6.
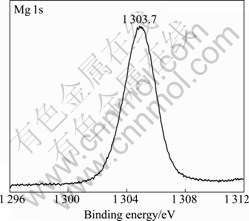
Fig.5 XPS spectrum of Mg 1s peak
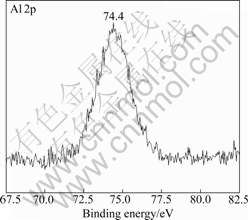
Fig.6 XPS spectrum of Al 2p peak
The XPS spectrum of O 1s was relatively wide compared with the standard O 1s peak, which implied that the peak might be a combination of oxygen in different status. Using standard data analysis procedures, the O 1s peak could be deconvoluted into two distinct peaks as shown in Fig.7. The first peak characterized at binding energy of 530.0 eV corresponded to the oxygen in metal oxide, and the second peak at a binding energy of 531.5 eV was attributed to the oxygen in metal hydroxide. XPS analysis results conformed that the corrosion product was composed of oxide and hydroxide of magnesium and aluminum.

Fig.7 XPS spectrum of O 1s peak
4 Conclusions
The atmospheric corrosion of magnesium AZ91D was influenced by NaCl deposition in humid air. The corrosion attack appeared localized characterization on AZ91 magnesium alloy. The corrosion rate was closely related to the exposure time of NaCl. The initial corrosion product provided protection for metal substrate and delayed further attack. The corrosion product was mainly a mixture of oxide and hydroxide of magnesium and aluminum.
References
[1] DECKER R F. Renaissance in magnesium [J]. Advanced Materials and Processes, 1998, 9: 31-33.
[2] ZENG Rong-chang, KE Wei, XU Yong-bo, HAN En-hou, ZHU Zi-yong. Recent development and application of magnesium alloys [J]. Acta Metallurgica Sinica, 2001, 37(7): 673-685. (in Chinese)
[3] SONG G L, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advanced Engineering Materials, 1999, 1(1): 11-33.
[4] MAKAR G L, KRUGER J. Corrosion of magnesium [J]. Int Mater Rev, 1993, 38(3): 138-145.
[5] SONG G L, ATRENS A, WU X L. Corrosion behavior of AZ21, AZ501 and AZ91 in sodium chloride [J]. Corrosion Science, 1998, 40(10): 1769-1791.
[6] AMBAT R, AUNG N N, ZHOU W. Evaluation of microstructure effect on corrosion behavior of AZ91D magnesium alloy [J]. Corrosion Science, 2000, 42(8): 1433-1455.
[7] YOUNG J F. Humidity control in the laboratory using salt solutions—A review [J]. Journal of Applied Chemistry 1967, 17(1): 241-245.
[8] LUNDER O, LEIN J E, AUNE T K, NISANCIOGLU K. Role of Mg17Al12 phase in the corrosion of Mg alloy AZ91 [J]. Corrosion, 1989, 45: 741-748.
[9] MATHIEU S, RAPIN C, STEINMETZ J, STEINMETZ P. Corrosion study of the main constituent phases of AZ91 magnesium alloys [J]. Corrosion Science, 2003, 45(12): 2741-2755.
[10] LINDSTROM R, JOHANSSON L G, THOMPSON G E, SKELDON P, SVENSSON J E. Corrosion of magnesium in humid air [J]. Corrosion Science, 2004, 46(5): 1141-1158.
[11] JONSSON M, PERSSON D, GUBNER R. Initial atmospheric corrosion of magnesium alloy AZ91D [J]. Journal of the Electrochemical Society, 2007, 154(3): C684-C691.
[12] LEBOZEC N, JONSSON M, THIERRY D. Atmospheric corrosion of magnesium alloys: Influence of temperature, relative humidity, and chloride deposition [J]. Corrosion, 2004, 60(4): 356-361.
[13] JONSSON M, PERSSON D, THIERRY D. Corrosion product formation during NaCl induced atmospheric corrosion of magnesium alloy AZ91D [J]. Corrosion Science, 2007, 49(3): 1540-1558.
[14] JONSSON M, THIERRY D, LEBOZEC N. The influence of microstructure on the corrosion behavior of AZ91D studied by scanning Kelvin probe force microscopy and scanning Kelvin probe [J]. Corrosion Science, 2006, 48(5): 1193-1208.
[15] LINDSTROM R, SVENSSON J E, JOHANSSON L G. The influence of carbon dioxide on the atmospheric corrosion of some magnesium alloys in the presence of NaCl [J]. Journal of the Electrochemical Society, 2002, 149(4): B103-B107.
Foundation item: Projects(50671005, 50971093) supported by the National Natural Science Foundation of China; Project(2007CB613705) supported by the National Basic Research Program of China
Corresponding author: ZHOU Wan-qiu; Tel: +86-24-86593312; E-mail: wqzhou @imr.ac.cn


