文章编号:1004-0609(2009)04-0754-06
从超富集植物Berkheya coddii中回收镍
杨建广1, 2,杨建英3,彭长宏1,唐朝波1,唐谟堂1,周科朝2
(1. 中南大学 冶金科学与工程学院,410083 长沙;
2. 中南大学 粉末冶金国家重点实验,410083 长沙;
3. 江西省环境工程职业学院,342000 赣州)
摘 要:
提出了超富集植物的“焚烧→湿法提取与净化→电化学沉积/化学沉淀法→金属/化工产品”工艺。以镍的超富集植物Berkheya coddii为例,对该收获物进行焚烧处理后,对焚烧产物进行氨法浸出,对浸出液进行一定处理后直接进行电积镍,最终得到高纯镍板(99.999%),该工艺既实现了对超富集植物收获物的去毒化处理,又实现了收获物中有价金属的回收。对于浸出实验,优化实验条件分别为:浸出温度60 ℃,氯化铵及氨水的摩尔比(n(NH4Cl)?n(NH3))0.6,液固比(L/S)5?1,浸出时间2 h;对于镍电积实验,优化实验条件为:ρ(Ni2+)浓度23 g/L,极距3.5 cm;电流密度200 A/m2,温度40 ℃。
关键词:
超富集植物;Berkheya coddii;资源化利用;浸出;电沉积;
中图分类号:TQ 134. 3 + 2 文献标识码: A
Recovery nickel from Berkheya coddii biomass
YANG Jian-guang1, 2, YANG Jian-ying3, PENG Chang-hong1, TANG Chao-bo1, TANG Mo-tang1, ZHOU Ke-chao2
(1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
3. Environmental Engineering Vocational College, Gangzhou 342000, China)
Abstract: The leaching and electrowinning of nickel from Berkheya coddii biomass was studied according to incineration→hydro-extraction and purification→electrodeposition→Metal/product flowsheet, and ammonia-ammonium chloride solution was used as leaching agents. The research was carried out in two phases, which are a leaching study to determine nickel extraction efficiency of this leaching agent, and an electrowinning study to determine nickel electrodeposition efficiency from the filtrate. The results show that leaching temperature has the most dominant effect on the metal extraction performance, followed by mole ratio of NH4Cl to NH3, liquid-solid ratio and leaching time. Accordingly, the optimum leaching conditions are that temperature of 50 ℃, n(NH4Cl)?n(NH3)=0.6, leaching time of 2 h and L/S=5?1. The total nickel removal after leaching under optimum condition reaches to 97.61%. The optimum conditions of nickel electrodeposition experiments are obtained as ρ(Ni2+) of 23 g/L, polar distance of 3.5 cm, current density of 200 A/m2 and temperature of 40 ℃, and the ultimate nickel content in the as-synthesized nickel plate is more than 99.999%.
Key words: hyperaccumulator; Berkheya coddii; resource utilization; leaching; electrowinning
植物修复技术以其治理效果的永久性、治理过程中的原位性、治理成本的低廉性和环境美学的兼容性等特点,目前已逐渐发展成为土壤污染治理的主要途径之一,并开始进入产业化初期阶段[1-4]。在我国本土已也发现蜈蚣草、大叶井口边草、东南景天和商陆等多种超富集植物。与此同时,国内外涉及超富集植物内容的文献也迅速增长[5-6]。但从目前已发表的文献来 看[7-10],研究焦点主要集中在超富集植物的筛选、鳌合诱导修复、接种菌根强化、基因技术、农艺管理和田间措施等[11-12]。而有关超富集植物收获物的处理及资源化技术,国内外的研究报道很少,且基本上都是实验室的简单探索。
超富集植物收获物的处置方法主要为焚烧法、堆肥法、压缩填埋法、高温分解法、灰化法和液相萃取法等。这些处理方法均存在设备投资大、二次污染环境、未能实现减量化及资源化利用等问题。考虑到目前超富集植物生物量逐年加大,而采用以上提及的现有处理方法有可能对人类造成潜在的危险,本研究以镍的典型超富集植物菊科的Berkheya coddii[13-15]为例,结合在湿法冶金处理低品位矿物原料的多年研究成果[16-19],提出超富集植物的“焚烧→湿法提取与净化→电化学沉积/化学沉淀法→金属/化工产品”工艺,以期有效分离富集这类物料中的有害重金属,实现超富集植物收获物的安全处置和资源化。
1 实验
1.1 实验原料
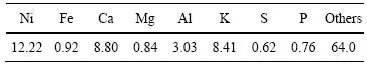
本次研究以江西环境工程职业学院提供的Berkheya coddii收获物为原料进行资源化处理,先将该原料进行焚烧,焚烧所用设备为德国马丁公司SITY 2000机械炉排焚烧炉,得到的焚烧产物的主要化学成分如表1所列。分析其中的镍物相,发现大部分镍(纯度大于95%)以镍的氧化物形态存在。
表1 Berkheya coddii 焚烧产物的主要成分
Table 1 Main chemical components of this biomass (mass fraction, %)
1.2 实验流程及方法
本研究提出的超富集植物收获物“焚烧→湿法提取与净化→电化学沉积/化学沉淀法→金属/化工产品”工艺流程示意图如图1所示。本次研究主要是对Berkheya coddii收获物焚烧产物的浸出、净化、净化液电积等3个过程进行研究。考察不同的实验条件对金属的浸出率、电积过程的电流效率及最终电积镍质量的影响。
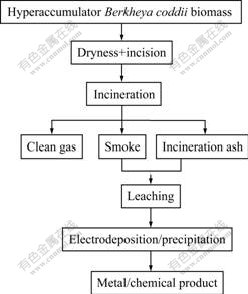
图1 超富集植物收获物资源化利用工艺流程示意图
Fig.1 Flow sheet of resource utilization of hyperaccumulator harvest
浸出实验采用正交试验设计,实验在500 mL三口平底烧瓶中进行,规模为每次50 g焚烧灰分,在三口烧杯的中间出口处设置一冷凝管,另外两个出口分别套上温度计及橡皮塞以减少溶液的蒸发损失。综合条件实验在1 L三口平底烧瓶中进行,规模为每次50 g焚烧灰分,实验采用恒温磁力搅拌仪搅拌,各实验的搅拌速度保持为200 r/min不变。考察有关因素对镍浸出率的影响。扩大试验在3 L三口平底烧瓶中进行,规模为每次500 g焚烧灰分。
在浸出净化液的镍电积试验中,每次试验取净化液2 L进行镍电积试验。以辽宁锦西化工研究所产涂钌钛板为阳极,有效面积为9 cm×10 cm,以纯钛板为阴极,有效面积为10 cm×10 cm,在2.5 L的玻璃烧杯中进行,用恒温磁力搅拌器控制温度。
1.3 分析方法
原料中的主要化学成分按照欧洲标准EPA Method 3052采用ICP-AES进行确定[20]。实验中所用的去离子水等按照欧洲标准EPA Method 3015进行制备[20]。浸出实验及镍电积试验中镍的含量用滴定法确定,电积镍板中杂质元素含量由ICP-AES法测定。
2 结果及分析
2.1 氨法浸出试验
氨法浸出实验采用正交试验法(L934)安排进行,正交实验因素及水平如表2所列,正交实验结果及其极差分析如表3所列。镍的浸出率方差分析如表4所列。
表2 Berkheya coddii 焚烧产物浸出正交实验因素及水平
Table 2 Experimental factors and levels selected for leaching of ground Berkheya coddii

表3 正交实验结果及其极差分析
Table 3 Leaching experiment and analysis of means (ANOM) and range
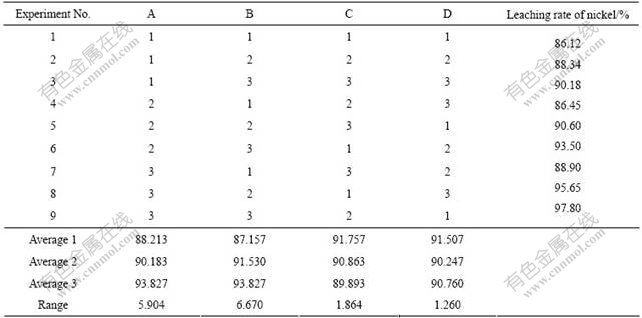
表4 镍浸出率的方差分析(α=0.05)
Table 4 Variation analysis of square error for nickel leaching rate

由表3和4可知,所选的NH3-NH4Cl-H2O体系可有效地分离Berkheya coddii收获物焚烧产物中的镍,并使其中大部分镍(最高达97.8%)被浸出而进入溶液。最佳浸出条件为:A3B3C1D3,即nNH4Cl ? nNH3(氯化铵及氨水的摩尔比)为0.6、反应温度50 ℃,L/S为 5?1,反应时间2 h。各工艺因素对镍浸出率的影响大小的顺序为浸出温度、n(NH4Cl)?n(NH3)、液固比、浸出时间,其中浸出温度及n(NH4Cl) ? n(NH3)为显著因素。
在以上最佳浸出条件下开展了3次规模为50 g 每次的综合试验,及3次规模为500 g 每次的放大试验。试验结果分别如表5和6所列。从表5可知,3次综合试验均很好地再现了优化条件的结果,镍的平均浸出率达到97.61%。而从表6可以看出,扩大试验在试验规模放大10倍后也很好地再现了综合条件的试验结果。分析浸出液中的主要化学成分,发现浸出液中主要含镍离子(23.5 g/L),而其它金属离子的含量较低,尤其是对后续电积过程影响较大的Fe、Al、Ca和Mg等进入浸出液的量较少,避免了电积前的溶液净化过程。NH3-NH4Cl-H2O 体系对物料的选择性浸出能力是其它酸性浸出体系所不能达到的,这也是本研究采用碱性体系对焚烧灰分进行处理的原因之一。
表5 综合试验结果
Table 5 Confirmation experiment results
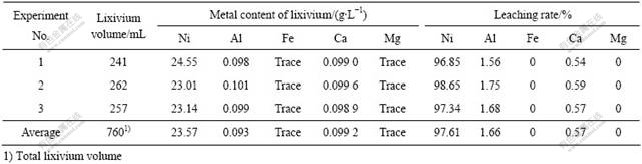
表6 扩大试验结果
Table 6 Scale-up experiment results
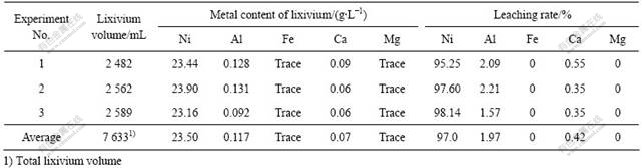
2.2 镍电积实验
将3次电积试验所得的浸出液合并(7.633 L,溶液主要成分为(g/L):Ni 23.5, Al 0.117,Ca 0.07),并往该溶液中加入一定量的水、NiCl2?6H2O、氯化铵、及氨水,以配制电解液的镍离子浓度为35 g/L,氯化铵5 mol/L,氨1.4 mol/L,溶液体积20 L,每次实验取上述电解液2.5 L开展电积实验。
2.2.1 Ni2+浓度对电流效率和电能消耗的影响
固定电流密度200 A/m2,极距3.5 cm,温度为 40 ℃,进行镍电积实验。每隔3 h取电积液一次,分析溶液中Ni2+浓度。Ni2+浓度对电流效率和电能消耗的影响如图2所示。
从图2可以看出,镍离子浓度对电流效率和电耗的影响较大,当镍离子浓度为18~23 g/L时,电流效率较高;当镍离子低于18 g/L时,电流效率明显减小;当镍离子达到23 g/L之后,电流效率趋于平衡。因此,最佳的镍离子浓度应控制在大于18 g/L。
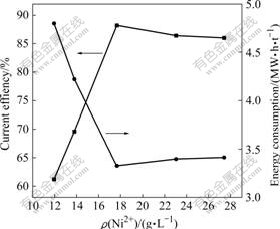
图2 Ni2+浓度对电流效率和电能消耗的影响
Fig.2 Effect of nickel ions concentration on current efficiency and energy consumption
2.2.2 不同电流密度下,极距对槽电压的影响
在其它条件不变的情况下,分别固定极距为2、3.5、5和7 cm,测定电流密度为200、250、300、350、400、450和500 A/m2条件下的槽电压。在不同电流密度下,极距对槽电压的影响如图3所示。

图3 不同电流密度下极距对槽电压的影响
Fig.3 Effect of polar distance on cell voltage under different current densities
从图3可以看出,电流密度和极距越大,槽电压也越大,电流密度对槽电压的影响更为明显。综合考虑,选定控制电流密度为200 A/m2,极距为3.5 cm。
2.2.3 温度的影响
固定极距为3.5 cm,电流密度为200 A/m2 ,在温度为30、40、50、60和70 ℃下进行电积实验,所得到的温度对电流效率和电能消耗的影响如图4所示。
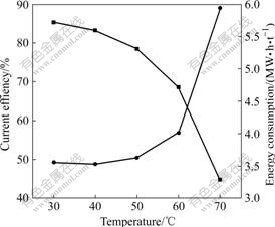
图4 温度对电流效率和电能消耗的影响
Fig.4 Effect of temperature on current efficiency and energy consumption
从图4可以看出,随着电解温度升高,尤其是当电解温度大于40 ℃后,电流效率降低非常明显,电能消耗急剧上升,因此,选定电解温度为40 ℃。
2.2.4 电流密度的影响
固定极距为3.5 cm,温度为40 ℃,分别取电流密度为200、250、300、350、400、450、500 A/m2进行电积试验。电流密度对电流效率和电耗的影响如图5所示。

图5 电流密度对电流效率和电耗的影响
Fig.5 Effect of current density on current efficiency and energy consumption
从图5可以看出,电流密度对电流效率和电耗的影响较为平缓,但当电流密度大于350 A/m2以后,电流效率明显下降;而当电流密度大于200 A/m2时,电流效率达最大值,但因电流密度太小,使试验时间过长,因此,确定电流密度应控制在200~300 A/m2之间。
2.3 镍电积综合实验
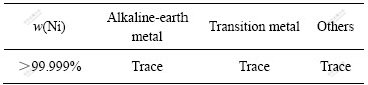
由上述单因素实验可得Ni(Ⅱ)-NH3-NH4Cl-H2O体系电积镍的优化试验条件为:ρ(Ni2+)浓度23 g/L,极距3.5 cm,电流密度200 A/m2,温度40 ℃。在此优化条件下进行3次镍电解实验,测得平均电流效率为90.51%,电压3.2 V。所得电镍质量经ICP-AES分析如下表7所列。
表7 电积镍主要成分分析
Table 7 Main chemical components of as-synthesized electrowinned nickel
3 结论
以镍的超富集植物Berkheya coddii为例,采用“焚烧→湿法提取与净化→电化学沉积/化学沉淀法→金属/化工产品”工艺对Berkheya coddii焚烧产物进行了处理。
2) 浸出试验的优化实验参数为浸出温度60 ℃;氯化铵及氨水的摩尔比(n(NH4Cl)?n(NH3))0.6;液固比(L/S)5?1;浸出时间2 h;
3) 镍电积试验,优化实验参数为:ρ(Ni2+)23 g/L;极距3.5 cm;电流密度200 A/m2;温度40 ℃,可最终得到99.999%的高纯镍。
REFERENCES[1] KR?MER U. Phytoremediation: novel approaches to cleaning up polluted soils[J]. Current Opinion in Biotechnology, 2005, 16: 133-141.
[2] GONZ?LEZ R C, GONZ?LEZ-CH?VEZ M C A. Metal accumulation in wild plants surrounding mining wastes[J]. Environmental Pollution, 2006, 144: 84-92.
[3] BAKER A J M, BROOKS R R, Terrestrial higher plants which hyperaccumulate metallic elements —a review of their distribution, ecology and phytochemistry[J]. Biorecovery, 1989, 1: 81-126.
[4] MIN Y, BOQING T, MEIZHEN T, AOYAMA I. Accumulation and uptake of manganese in a hyperaccumulator phytolacca americana[J]. Minerals Engineering, 2007, 20: 188-190.
[5] SUN B, ZHAO F J, LOMBI E, MCGRATH S P. Leaching of heavy metals from contaminated soils using EDTA[J]. Environmental Pollution, 2001, 113: 111-120.
[6] BROWN S L, CHANCY R L, ANGLE J S, BAKER A J M, Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens and metal tolerant silene vulgaris grown on sludge-amended soils[J]. Environmental Science and Technology, 1995b, 29(6): 1581-1585.
[7] M SRIVASTAVA, MA L Q, ANTONIO J. Three new arsenic hyperaccumulating ferns[J]. Science of the Total Environment, 2006, 364: 24-31.
[8] IDRIS R, KUFFNER M, BODROSSY L, PUSCHENREITER M. Characterization of Ni-tolerant methylobacteria associated with the hyperaccumulating plant Thlaspi goesingense and description of Methylobacterium goesingense sp. nov.[J]. Systematic and Applied Microbiology, 2006, 29: 634-644.
[9] MC GRATH S P. Phytoextraction for soil remediation in Plants that hyperaccumulate heavy metals[M]. Oxon UK: Wallingford, 1998: 261-287.
[10] ROBINSON B H, LEBLANC M, PETIT D, BROOKS R R, KIRKMAN J H, GREGG P E H. The potential of Thlaspi caerulescens for phytoremediation of contaminated soils[J]. Plant Soil, 1998, 203: 47-56.
[11] CATHER I, CHRISTIAN L. Thermal treatment of metal- enriched biomass produced from heavy metal phytoextraction[J]. Environmental Science and Technology, 2005, 39: 3359-3367.
[12] ZHAO F J, LOMBI E, BREEDON T. Zinc hyperaccumulation and cellular distribution in Arabidopsis haller[J] Plant Cell and Environment, 2006, 23: 507-514.
[13] HARRIS A T, NAIDOO K, NOKES J, WALKER T, ORTON F. Indicative assessment of the feasibility of Ni and Au phytomining in Australia[J]. Journal of Cleaner Production, 2009, 17: 194–200.
[14] LIEVENS C, YPERMAN J, VANGRONSVELD J, CARLEER R. Study of the potential valorisation of heavy metal contaminated biomass via phytoremediation by fast pyrolysis: Part I. Influence of temperature, biomass species and solid heat carrier on the behaviour of heavy metals[J]. Fuel, 2008, 87: 1894-1905.
[15] AUGUSTYNIAKA M, MIGULAA P, MESJASZ- PRZYBY1OWICZB J, TARNAWSKA M. Short-term effects of dimethoate on metabolic responses in chrysolina pardalina (chrysomelidae) feeding on Berkheya coddii (asteraceae), a hyper-accumulator of nickel[J]. Environmental Pollution, 2007, 150: 218-224.
[16] YANG Sheng-hai, TANG Mo-tang, CHEN Yi-feng, TANG Chao-bo, HE Jing. Anodic reaction kinetics of electrowinning system of Zn(Ⅱ)-NH3 -NH4Cl-H2O[J]. Trans Nonferrous Met Soc China, 2004, 14(3): 626-630.
[17] TANG Mo-tang, YANG Jian-guang, YANG Sheng-hai, TANG Chao-bo. Thermodynamic calculation of Sn(IV)-NH4+-Cl--H2O system[J]. Trans Nonferrous Met Soc China, 2004, 14(4): 802-806.
[18] 杨建广, 唐谟堂, 杨声海, 唐朝波. 配合-共沉淀法制备锑掺杂二氧化锡(ATO)粉[J]. 中国有色金属学报, 2005, 15(6): 966-974.
YANG Jian-guang, TANG Mo-tang, YANG Sheng-hai, TANG Chao-bo. Transparent conductive oxide ATO powders prepared by complex-co-precipitation method[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(6): 966-974.
[19] 杨建广, 唐谟堂, 杨声海. Sn(Ⅳ)-Sb(Ⅲ)-NH3-NH4Cl-H2O体系热力学分析及其应用[J]. 中南大学学报(自然科学版), 2005, 36(4): 582-586.
YANG Jian-guang, TANG Mo-tang, YANG Sheng-hai. thermodynamics analysis of Sn(Ⅳ)-Sb(Ⅲ)-NH3-NH4Cl-H2O system and its application[J]. The Journal of Central South University (Science and Technology), 2005, 36(4): 582-586.
[20] Environmental protection Agency. Methods for analytes and properties[M]. United States: Environmental Protection Agency, 1998.
基金项目:国家高技术研究发展计划资助项目(KY20080577000002);国家自然科学基金资助项目(50804056;50774099);中国博士后基金资助项目(20080431028)
收稿日期:2008-10-08;修订日期:2008-12-18
通讯作者:杨建广,副教授,博士;电话:0731-8830470;E-mail: Jianguang_yang@hotmail.com
摘 要:提出了超富集植物的“焚烧→湿法提取与净化→电化学沉积/化学沉淀法→金属/化工产品”工艺。以镍的超富集植物Berkheya coddii为例,对该收获物进行焚烧处理后,对焚烧产物进行氨法浸出,对浸出液进行一定处理后直接进行电积镍,最终得到高纯镍板(99.999%),该工艺既实现了对超富集植物收获物的去毒化处理,又实现了收获物中有价金属的回收。对于浸出实验,优化实验条件分别为:浸出温度60 ℃,氯化铵及氨水的摩尔比(n(NH4Cl)?n(NH3))0.6,液固比(L/S)5?1,浸出时间2 h;对于镍电积实验,优化实验条件为:ρ(Ni2+)浓度23 g/L,极距3.5 cm;电流密度200 A/m2,温度40 ℃。


