
Biocompatibility and preliminary clinical application of HA/HDPE nanocomposites synthetic auditory ossicle
ZHU Shai-hong(朱晒红)1, WANG Guo-hui(王国慧)1, ZHAO Yan-zhong(赵颜忠)1, QI You-fei(戚悠飞)1,
ZHOU Ke-chao(周科朝)2, HUANG Su-ping(黄苏萍)2, LI Zhi-you(李志友)2, HUANG Bai-yun(黄伯云)2
1. Transplantation Medical Academy, The Third Xiang-Ya Hospital, Central South University, Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 20 April 2006; accepted 30 June 2006
Abstract:
The biocompatibility of the hydroxyapatite/high density polyethtlene(HA/HDPE) nanocomposites synthetic auditory ossicle was evaluated, the percentage of S-period cells was detected by flow cytometry after L929 incubated with extraction of the HA/HDPE nanocomposites, titanium materials of clinical application as the control. Both of them were implanted in the animals and the histopathological evaluations were carried out, and the preliminary clinical trials about HA/HDPE nanocomposites synthetic auditory ossicles were also carried out. The statistical analysis show that there are no statistically significant differences between HA/HDPE test groups and control groups (P>0.05), which demonstrates that the HA/HDPE nanocomposites synthetic auditory ossicle has a good biocompatibility and clinical application outlook.
Key words:
hydroxyapatite; high density polyethtlene; ossicle; biocompatibility; flow cytometry;
1 Introduction
Ossicular replacement prosthesis ideally should have a good biocompatibility, all the occurrence of the good reaction, not bulge, not absorbability, the long-term stability of characteristic, have no influence on sound conduction. Looking for the ossicular prostheses ideally has always been a hot spot of otology research. Now clinical applied such ossicular replacement prosthesis metals as titanium, hydroxyapatite, polyethylene and so on. In recent years, composite materials comprised of bioactive inorganic nanoparticles and organic polymers have been studied, it integrates some properties including good bioactivity of porcelain and ceramics, bone conductibility and good mechanics characteristics of metals and the polymer, and have become one of the most viable research directions of ossicular replacement prosthesis biomaterials currently[1-4]. However, new applications of biomaterials require testing to ensure safety and efficacy. Testing needs to be designed for specific implant requirements and varies widely according to implant type[5]. The aim of this study is to evaluate the biocompatibility of the composite materials for synthetic auditory ossicle, the hydroxyapatite/high density polyethtlene (HA/HDPE) nanocomposites. Compared with the titanium applied in clinic according to GB/ T16886[6], the percentage of S-period cells was detected by flow cytometry after L929 incubated with extraction of HA/HDPE nanocomposites, the histopathological evaluations were carried out after the materials implanted in the animals. And the preliminary clinical trials about HA/HDPE nanocomposites synthetic auditory ossicle were carried on.
2 Experimental2.1 Preparation of materials
Clinical titanium implants were control groups; HA/HDPE synthetic auditory ossicle (Provided by State Key Laboratory of Power Metallurgy, Central South University) were experimental groups.
Preparative methods: Hydroxyapatite and high density polyethtlene nano-powder were mixed, then sintered at 220 ℃ for 2 h and extruded at 140 ℃ solid phase with forcing machine. At last HA/HDPE nanocomposites were obtained while it was cooling.
Sterilization procedures: The implants were cleaned in trichlorethylene (99.5%), rinsed in absolute ethanol, and autoclaved (120 ℃) for 30 min to ensure the sterility of samples.
2.2 Cytotoxicity experiments
2.2.1 Reagents and instruments
L929 mouse skin fibroblasts were provided by Xiang-Ya Medical School, Central South University, fetal calf serum was purchased from Siji Qing Co. of Hangzhou, RPMI-1640 and trypsinase were purchased by GIBCO Co. and PI was purchased by Sigma Co., the rest reagents were analytical reagents. Inverted phase- contrast microscope, CO2 incubator, FACSC calibur flow cytometry (BD Co.) and six healthy young swines were provided by the Experimental Animal Center of Xiang-ya Medical School.
2.2.2 Preparation of extract liquid
Clinical titanium implants and HA/HDPE synthetic auditory ossicles were steam sterilized at 137.3 kPa, 121 ℃ for 30 min and RPMI-1640 medium in culture flask according to the ratio of 0.2 g/mL between quality of materials and volume of culture medium, then the flask were cultured about 72 h in humidified incubator at 37 ℃ with 5% CO2 in air.
2.2.3 Cell culture
L929 mouse skin fibroblasts were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum about 24 h in humidified incubator at 37 ℃ with 5% CO2 in air, and passage after the cell overgrew culture flask.
2.2.4 Flow cytometry assay
L929 mouse skin fibroblasts were serial subcultivated 24 h,and the medium of each culture flask was replaced respectively with leaching liquor of two materials. These culture flasks were incubated 24 h again at the same condition and were observed by inverted microscope. After the cells attached on the bottom of the culture flask, living cells were collected by trypsinization and were washed twice with PBS(phosphate buffer saline) buffer solution. Then, the living cells were fixed with 4 ℃,70% alcohol, centrifuged at 500-1 000 r/min for 5 min, the supernatants were discarded, and the cells were suspended for 5 min in buffer solution, centrifuged again and discarded supernatants. The cells were stained with PI( propidium iodide )to make the final concentration to 100 ?g/mL, then the cells were protected from light at 4 ℃ for 30 min.
The cells were analyzed using a FACscan (Becton-Dickinson) and LYSIS II software. Cells were initially selected based on morphological criteria (Fas and Ras parameters) by sieving with 400 meshes, which allowed us to exclude cell debris and aggregates. Only isolated, intact cells were analyzed.
2.3 Implant experiment in vivo
All experimental animal procedures were approved and monitored by the Academic Committee of Xiang-ya Medical School of Central South University, and performed according to the institutional guide-lines on the care and use of laboratory animals. A total of 15 specific pathogen free young swines weighing 20-25 kg were used. 5 swines underwent surgical procedures as an experimental implant group, 5 swines underwent the same procedures as a control implant group, and 5 swines underwent sham operations as a negative control group. Surgery was carried out under general anesthesia using 2% pentobarbital under sterile conditions. The abdominal wall of the animals were shaved and disinfected with 5 % iodine in ethanol. An incision from the animals’ abdominal wall to abdominal wall muscular layer was made in 2 cm two sides of medioventral line, and chosed six implant spots and each spot between 2 cm, and implanted the two materials into spots respectively. Then, the incisions was closed with one or two stitches. Two swines in each group were sacrificed with an anodynia way at 14, 30 and 90 d after surgery.
Materials were removed along with 0.5-1.0 cm surrounding tissue and immersed in 4% buffered formalin. After fixing for 24 h, implants and surrounding implanted tissue were dissected and dehydrated in an ethanol series, embedded in paraffin and cut into approximately 10μm-thick sections. For each specimen, 10 to 15 sections were made and stained with haematoxylin and eosin (H & E).
For each specimen, five randomly chosen sections of the soft tissue surrounding the implants were observed and photographed (color slide) under a light microscope. Color slides (original magnification of 100) of cell distribution around implants were enlarged by projecting slides on a screen to identify and count the different cells. Between 180 and 240 cells were counted for each specimen and percentages of the various component cells were calculated.
2.4 Statistics
The software package SPSS 11.0 was used for statistical analysis. The data are reported as mean ± standard deviation (SD). Student’s t-test was performed for analysis in this report. Statistical significance was established at P<0.05.
2.5 Preliminary clinical trials
2.5.1 Clinical data
To evaluate the clinical effect of HA/HDPE synthetic auditory ossicle in tympanoplasty, tympanoplasty in intact canal was performed in 10 ears (7 patients) with HA/HDPE synthetic auditory ossicle, and all patients of them were followed up with an average period of 1.5 a, the longest period was 3 a.
2.5.2 Surgical operation methods
The open tympanoplasty was performed in 7 patients with chronic otitis media under general anesthesia through a postauricular cut. After complete skeletonizing and exenteration in tympanic cavity, reconstructed ossicular chain with HA/HDPE synthetic auditory ossicle on the basis of the eustachian tube function permission.
3 Results
3.1 Cytotoxicity experiments
The flow cytometry results following the 24 h contact period showed that the cell cycle of Material HA/HDPE group was essentially the same for Material Titanium group and negative RPMI1640 culture medium group. They show that (49.86±4.28)% of the cells in contact with Material HA/HDPE group were in the S-phase (Table 1 and Fig.1).
Table 1 Percentage of S-period cells (%)
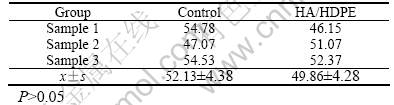
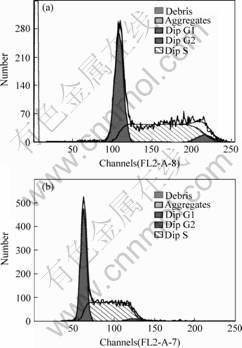
Fig.1 Graphs of cell cycle of control groups(a) and HA/HDPE groups(b)
Materials HA/HDPE and material titanium have approximately the same percentage of S-phase cells compared to the negative RPMI1640 culture medium group), (49.86 ± 4.28)% and (52.13 ± 4.38)%, respectively. Cell distribution in the cycle is approximately equal. The proliferation index (percentage of S-phase cells) is not statistically different between Materials HA/HDPA and Material Titanium and the negative RPMI1640 culture medium control (P>0.05).
3.2 Implant experiment in vivo
The tissue around the implants had two main structures, a layer of acute inflammatory cells, such as neutrophils, macrophages and lymphocytes, and a surrounding layer of fibrous tissue composed of fibroblasts and fibrocytes, and in some cases capillaries after 14, 30, 90 d implantation.
Specimens obtained 14 d after implantation showed that macrophages and lymphocytes remained at a low level. The implants were surrounded by a thin layer of fibroblasts and fibrocytes. Cellular reactions and the degrees of neutrophils infiltration to HA/HDPE synthetic auditory ossicles were similar to the reactions in the negative control groups at 14 d. The degree of neutrophils infiltration to material titanium was 23.5±0.05, material HA/HDPE was 26.2±0.14. The degrees of lymphocytes infiltration to Material Titanium was 10.9±0.81,and that of material HA/HDPE was 12.8±0.45(Fig.2). There were no statistically significant differences (P>0.05) between the numbers and types of cellular components and the degrees of neutrophils infiltration with respect to implantation site. From 14 d to 90 d, cells around implants were mainly fibroblasts and fibrocytes, which reflected the stability of HA/HDPE synthetic auditory ossicles in tissues. Experimental findings suggested that these two kinds of biosynthesis materials had satisfactory biocompatibility.
3.3 Initial clinical trials
Of the 7 cases followed up, the average of air conduction before operation was 60.16 dB HL and after operation was 34.66 dB HL, hearing raised evenly 25.5 dBHL. 5/7 (71.4%) had regained socially acceptable hearing ability. No reject reaction and recurrent cholesteatoma were found(Fig.3).
4 Discussion
A better property can be obtained if biomaterial is more similar to natural tissue. Hydroxyapatite contains the main inorganic constituents of mature bone[7], providing optimal biocompatibility as a bone replacement in otolaryngo logical and other surgeries,
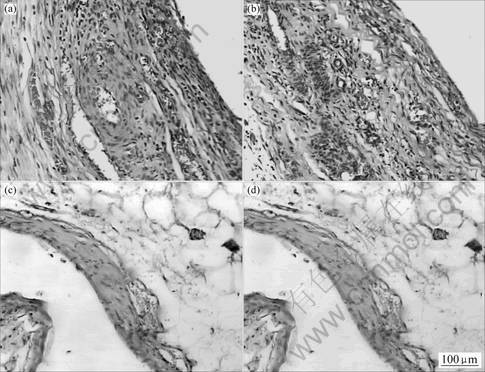
Fig.2 Graphs of tissue pathological section of control groups(a), (c) and HA/HDPE groups(b), (d) in 14 and 30 d

Fig.3 Graphs of HA/HDPE synthetic anditory ossicle
such as implantation of a synthetic auditory ossicle. The HA nanocomposite crystal used in this study were similar to bone apatite crystals in morphology, phase composition and crystal structure. The high HA content in composite offsets the bioinert of polyethtlene. The use of HA and HDPE contributes to the enhancement of mechanic property. The HA/HDPE composite provides an opportunity to produce biomimetic materials for clinical applications[8,9].
Biocompatible materials are used to enhance, treat or replace organs or organ functions and, of necessity, enter into contact with living tissues. In vitro testing, while a single step, is nonetheless vital to study the biocompatibility of a material. This testing must provide information about cell death, adherence and proliferation as well as about morphological changes and biosynthetic activity [10]. The method of cell culture in vitro is a quick and effective toxicity screening program[11]. It is a quick and precise method to evaluate the effect of different biomaterials on cytoactive by detecting S-period cells with flow cytometry after the cell cultured with materials[12]. The higher the percentage of S-period cells were, the less the toxicity of biomaterials was[13]. The detection of cell cycle may become a reliable method for evaluation of material biocompatibility.
Various biomaterials are being widely used in clinical practice[1-4]. Knowledge of how a material interacts within the environment of the body, particularly at the implant body interface and in the surrounding tissues, is necessary to determine its applicability as an implant material. One factor that determines the success of an implant is the inflammatory reaction in vivo [1,2,5,6]. The tissue reactions observed in the present study were similar to other experimental and clinical studies of hydroxyapatite implantation in vivo, which reported low levels of inflammation and fibrosis. It is well documented that HA/HDPA materials are highly compatible and non-immunogenic with host tissues.
5 ConclusionsHA/HDPE material has little cytotoxicity in vitro and little inflammatory reaction in vivo, and it is insignificantly different from titanium implants in clinical application. Good biocompatibility for HA/HDPE material in vivo suggests that HA/HDPA synthetic auditory ossicle is suitable for implantation in ossiculoplasty.
AcknowledgementsThe authors thank the financial support of the Ministry of Science and Technology of China and the National High-Tech Commission of China.
References[1] HIENDERANER G, MCGEE T, KUDEJ R. Evaluation of a bioactive ceramec composite as a dental implant[J]. Ceramic Bull, 1991, 70: 1010-1015.
[2] PERRY A C. Integrated orbital implants[J]. Adv Ophthalmic Plast Reconstr Surg,1990, 8: 75-81.
[3] de GROOT K,GEESINK R C T,K1EIN C P A T, et al. Plasma-sprayed coatings of hydroxyapatite[J]. J Biomed Mater Res, 1987, 21: 1375-1381.
[4] RABALAIS M L, Jr YUKNA R A, MAYER E T. Evaluation of durapaite ceramic as an alloplastic implant in periodontal osseous defect (I)-Imitial six-month results[J].J Periodontal, 1981, 11: 680-689.
[5] de MANE C Q. The development of implant and implantable materials[J]. Otolaryngol Clin North Am, 1995, 28: 225-234.
[6] YU Y T, ZHANG X D. Biomedical Materials[M]. Tianjin: Tianjin University Press, 2000. 28-34.
[7] HULBERT S F, BOKROS J C, HENCH L L, et al Ceramics in clinical applications, past, present and future [A]. Vincenzini P. High Tech Ceramics [M]. Milan: Milan Press, 1986. 189-190.
[8] FU T, YU D M. Compounding techniques of bioactive ceramic and polymer biomaterials[J]. Journal of Biomedicine Engineering, 2002, 19(1): 108-111.
[9] WANG Xue-jiang, LI Yu-bao, WEI Jie. Development of biomimetic nano hydroxyapatite/poly(hexamethylene adipamide)composites[J]. Biomaterials, 2002, 23: 4787-4791.
[10] KIRKPATRICK C J, MITTERMAYER C. Theorical and practical aspects of testing potential biomaterials in vitro[J]. J Mater Sci Mater Med, 1990, 1: 9-13
[11] STIVASTAVA S, STEPHEN D G.. Screening of in vitro cytoxicity by the adhesive test[J]. Biomaterial, 1990; 11(3): 133.
[12] WANG J Z, WANG S J. The tendency of development flow cytometry analyse at present[J]. Chin J Lab Med, 2002, 5(1): 5-7.
[13] LIU X L, ZHANG C X, WEN X L, et al, Study on correlation between in vitro and in vivo biocompatibility test of biomaterials-the flow cytometry method and the subcutaneous implant test[J]. Journal of Stomatology Material Apparatus, 1999, 8(3): 120-122.
Foundation item: Project(2003AA302210) supported by the Hi-Tech Research and Development Program of China
Corresponding author: ZHOU Ke-chao; Tel: +86-731-8836264; E-mail: zhushaihong@med.mail. com.cn


