
Composition of off-gas produced by combined fluidized bed chlorination for preparation of TiCl4
XIONG Shao-feng(熊绍锋)1, 2, 3, YUAN Zhang-fu(袁章福)1, 2, XU Cong(徐 聪)4, XI Liang(席 亮)3
1. State Key Laboratory of Multiphase and Complex Systems, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China;
2. Department of Energy and Resources Engineering, College of Engineering,
Peking University, Beijing 100871, China;
3. Graduate University of Chinese Academy of Sciences, Beijing 100049, China;
4. Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China;
Received 17 December 2008; accepted 25 March 2009
Abstract:
The effects of carbon/slag molar ratio, chloride amount and temperature on equilibrium molar ratio (REq) of CO to CO2 for off-gas produced by carbochlorination of titanium slag were firstly investigated by thermodynamic calculation of equilibrium components of off-gas. The experimental CO/CO2 molar ratio (REx) was then obtained to be 0.2-0.3 by the carbochlorination experiment using a novel combined fluidized bed as chlorination reactor. To further investigate the reaction effect of the novel process mentioned above, REx, REq and corresponding reference data (RRe) were compared. The results indicate that REx is similar to RRe (0.5-1.2) but different from REq (≥4.3), which is consistent with anticipation of REx for the novel combined fluidized bed. The difference between REx and corresponding REq is mainly attributed to short retention time (about 1 s) of materials in combined fluidized bed and carbochlorination of oxide impurities contained in titanium slag, such as CaO, MgO and SiO2.
Key words:
titanium slag; equilibrium component; combined fluidized bed; carbochlorination;
1 Introduction
Titanium resources from Panzhihua (Sichuan Province in China) are very rich and vanadic titanomagnetite reserves were proven to be 9.66×108 t, ranking first in the world. However, even after separation of iron and vanadium, 95% titanium resources cannot be used directly as the starting materials for preparation of titanium tetrachloride (TiCl4) by fluidized chlorination bed[1]. One of the major reasons is high total content of CaO and MgO impurities (6%-9%, mass fraction) beyond the suitable range of 0.5%-1.0% for the commercial production of TiCl4 to easily cause bed-agglomerate[1-3]. Several methods, such as chlorination in molten salt[4], chlorination in higher reaction temperature[4] and lower reaction temperature[5], have been proposed for anti-agglomeration. However, these methods were respectively confronted with inevitable problems in terms of environmental hazards, erosion of chlorinated reactor or increasing cost.
A method for anti-agglomeration without these shortcomings mentioned above was proposed using a novel combined fluidized bed as chlorination reactor[6-8]. A series of corresponding researches had been processed according to the combined fluidized bed, such as one-dimensional model for optimal technological parameters[6-7] and verification of effect of anti-agglomeration on an experimental scale[8]. However, the components, especially the CO/CO2 molar ratio, of off-gas released from chlorination fluidized bed need to be further investigated.
CO/CO2 molar ratio is one of the major parameters in the fluidized chlorination process, which reflects theoretical carbon consumption, thermal effect, etc[9]. Furthermore, CO/CO2 molar ratio is correlated with the efficiency of carbochlorination of TiO2. WANG and MA[9] compared CO/CO2 equilibrium molar ratio with that of off-gas produced by carbochlorination of titanium slag respectively corresponding to fluidized bed, molten salt chlorinating process and shaft furnace. However, the factors influencing the CO/CO2 molar ratio were not discussed roundly. LIN and LEE[10], and YAGI and OKUDAIRA[11] investigated the relationship of temperature and CO/CO2 molar ratio of off-gas produced by carbochlorination of rutile (w(TiO2)≥96%). However, the relationship may be not fit for the carbochlorination of titanium slag (w(TiO2)=78%-83%). Up to now, few studies have been devoted to the relationship of CO/CO2 molar ratio of off-gas and its equilibrium data under different technological conditions according to fluidized chlorination process of titanium slag. In addition, the relationship of CO/CO2 molar ratio with reaction effect, theoretical carbon consumption, thermal effect, etc., for conventional fluidized bed may be invalid to be employed as reference for the novel combined fluidized bed due to their constructional difference. CO/CO2 molar ratio for the novel fluidized bed needs to be further investigated.
In this study, the experimental CO/CO2 molar ratio (REx) was gained by carbochlorination of Panzhihua titanium slag in the novel combined fluidized bed. At the same time, HSC software was employed to calculate equilibrium components and the CO/CO2 equilibrium molar ratio (REq) under different technological conditions. REx was further compared with REq to evaluate reaction conversion.
2 Experimental
Panzhihua titanium slag with particle size of about 70-150 μm, as described in Table 1, was used as starting material. Petrocoke with particle size of about 500-850 μm, as described in Table 2, was provided by Panzhihua Steel & Iron Group, China, for premix with titanium slag. Off-gas discharged from TiO2-preparing working section was used as chloride and its composition is listed in Table 3.
Table 1 Composition of Panzhihua titanium slag (mass fraction, %)
![]()
Table 2 Chemical composition of petrocoke (mass fraction, %)
![]()
Table 3 Composition of oxidation off-gas (volume fraction, %)
![]()
Schematic diagram of the experimental technological process is shown in Fig.1. The reactor may be composed of one or more parts, each of which consists of a riser with the inner diameter of 22 mm and a semi-circulating fluidized bed (SCFB) with the inner diameter of 59 mm. K-type temperature thermocouple was employed. The mixture of titanium slag and petrocoke was added into the combined bed bottom at the solid materials speeding rate of 120-450 g/min. Other technological parameters were shown in previous work[6-8] including that temperature range was between 923.15 and 1 273.15 K and gas apparent velocity was between 0.7 and 1.1 m/s.
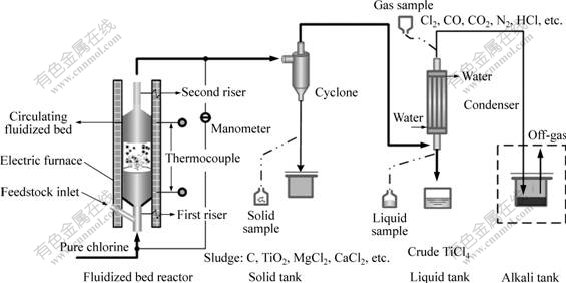
Fig.1 Schematic diagram of experimental process
Furnace slag was discharged regularly. Off-gas and powder discharged from top of combined fluidized bed and then entered condenser through cyclone for separating gases with different boiling points. The gases with low boiling point, such as TiCl4, SiCl4, VCl3 and VOCl3, became condensed and were collected in liquid tank for further purification. The gases with high boiling point, such as CO, CO2, N2, HCl and a small amount of Cl2, passed through condenser top where off-gas was sampled and further disposed in alkali tank apparatus before venting to the atmosphere.
The components of off-gas were measured by Orsat gas analyzer. The morphology and components of furnace slag were analyzed by JSM-646LV scanning electron microscope with an energy-dispersive spectrometer (SEM-EDS).
3 Thermodynamic calculation
There exist the following main reactions in the course of carbochlorination of titanium slag:
TiO2+2C+2Cl2=TiCl4+2CO (1)
TiO2+C+2Cl2=TiCl4+CO2 (2)
C+CO2=2CO (3)
Eq.(3) shows the gasification of carbon. Eq.(4) can be derived as follows by combination of Eqs.(1), (2) and (3), which totally expresses the quantitative relation of all the components:
TiO2+2(1+R)/(R+2)C+2Cl2=
TiCl4+2R/(R+2)CO+2/(R+2)CO2 (4)
where R is the CO/CO2 molar ratio of off-gas.
The carbochlorination reactions of oxide impurities in titanium slag, such as MgO and CaO, are shown as follows:
MgO+C+Cl2=MgCl2+CO (5)
2MgO+C+2Cl2=2MgCl2+CO2 (6)
CaO+C+Cl2=CaCl2+CO (7)
2CaO+C+2Cl2=2CaCl2+CO2 (8)
Chemical equilibrium calculations of the TiO2-C-Cl2 reaction system were performed by the HSC program (Version 1.1)[12]. This mechanism of the program is based on the minimization of Gibbs free energy. The HSC database consists of enthalpy (H), entropy (S) and heat capacity (C) data of above 5600 species mainly according to the handbook edited by BARNER and SCHEURMAN[13].
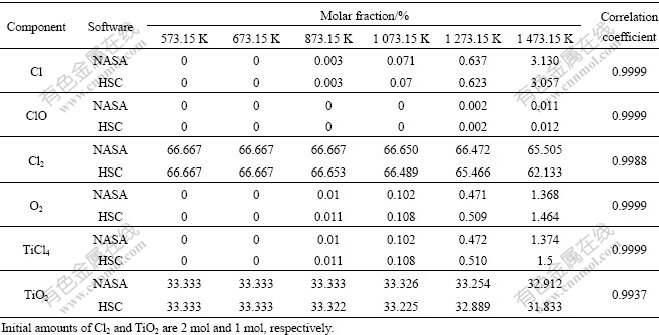
The calculation reliability of HSC software can be testified by comparison of the results calculated by HSC with the results simultaneously calculated by other software for a certain system. The equilibrium product components of directly chlorinated reaction of TiO2 were calculated herein by both HSC and NASA CEA program [5] and the results were compared, which are shown in Table 4. All the coefficients of correlation are close to 1, which indicates the reliability of HSC.
Table 4 Simulated equilibrium molar fractions of reaction system of Cl2 and TiO2 for direct preparation of TiCl4 at various temperatures
The chemical equilibrium calculations were processed to investigate the effects of temperature and molar ratio of starting materials on REq. The components of impurities in titanium slag were considered according to the components of Panzhihua titanium slag as described in Table 1. The effect of n(Cl2)?n(TiO2) was investigated by means that REq correlated to different n(C)?n(TiO2) in the range of 1.6?1 to 2.6?1 was calculated at constant n(Cl2)?n(TiO2). Similarly, the effect of n(Cl2)?n(TiO2) on REq was investigated.
4 Calculated results and discussion
4.1 Effect of temperature on equilibrium components and REq
In the thermodynamic point of view, reaction temperature is the main factor influencing the equilibrium state correlated to CO/CO2 molar ratio. Fig.2(a) shows the calculated result of equilibrium components under the conditions: n(Cl2)?n(C)?n(TiO2) of 2.0?2.2?1.0 and pressure of 102.1 kPa. The equilibrium molar fraction of CO2 and TiCl4 in off-gas was very high below 873 K and decreased with the enlargement of the temperature until undergoing the turning point where CO became dominant above 1 000 K. Therefore, REq increased with temperature in the range of 673-1 073 K. The result is consistent with the results given by ARTHUR[14] that the effect of reaction temperature on REq for carbochlorination of TiO2 was similar to that for the gasification of carbon.
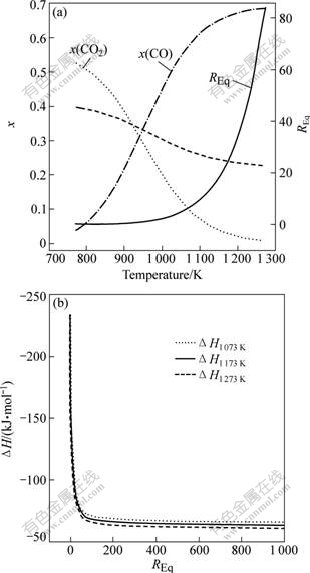
Fig.2 Effect of temperature on REq and equilibrium components of off-gas (a) and relationship between REq and reaction enthalpy corresponding to Eq.(4) (b)
Fig.2(b) shows the relationship between REq and reaction enthalpy corresponding to Eq.(4) at various temperatures. At first, it indicates that the thermal effects increased with increasing the temperature in the range of 1 073-1 273 K. In addition, the thermal effect of reaction totally occurred according to Eq.(2) (the ordinate for REq=0 as shown in Fig.2(b)) is apparently greater than that according to Eq.(1) (the ordinate for REq→+∽ as shown in Fig.2(b)). Therefore, the corresponding relationship between REq and reaction enthalpy may be revealed by a phenomenon that the reaction enthalpy decreases with increasing REq. It can be employed to explain the application of technique with ‘low REq’ for the purpose of energy-saving in practical production process, in which both carbon and titanium slags were reduced and even somewhat oxygen was pumped in chlorinator for enabling the carbochlorination of TiO2 to occur according to Eq.(3) with a low REq to produce a large amount of CO2 and maintain the furnace temperature.
4.2 Effect of carbon/slag ratio on REq
Carbon/slag ratio can be represented by n(C)?n(TiO2) and its effect on REq at different temperatures is shown in Fig.3. REq kept increasing with increasing n(C)?n(TiO2) from 1.5?1 to 2.2?1. The results indicate that the incomplete gasification caused by excess carbon, above 2?1, was one of the main reasons of the higher equilibrium molar fraction of CO in off-gas, which further aroused the increase of REq.
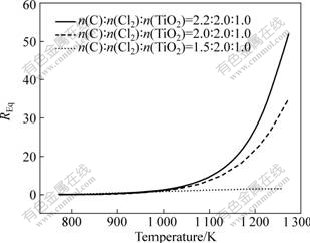
Fig.3 Effect of carbon/slag molar ratio on REq
4.3 Effect of chlorine/slag ratio on REq
Fig.4 shows the effect of n(Cl2)?n(TiO2) at various temperatures and initial n(C)?n(TiO2)=2.2?1.0 which is in excess of stoichiometry to facilitate a complete conversion of TiO2. REq increased simultaneously with the decrease of n(Cl2)?n(TiO2) in the range from 2.0?1.0 to 3.0?1.0.
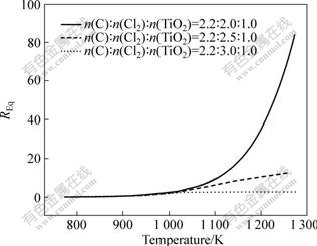
Fig.4 Effect of chlorine/slag molar ratio on REq
The effect of the ratio of carbon/slag and chlorine amount can be contributed to three main reasons. Firstly, the existence of oxide impurities in titanium slag led to variance of REq of off-gas in view that the CO/CO2 molar ratio of product gas is different from REq. Secondly, the initial molar ratio of CO to CO2 contained in oxidation chlorinate, about 0.28, would be different from REq in most cases, which had an influence on REq. The more the oxidation chlorinate pumped in fluidized bed, the greater the influence on REq. Thirdly, the carbochlorination of low-valent titanium oxide, such as TiO and Ti3O5, contained in titanium slag, was easier to occur according to SERYAKOV and BAKS[15] and COLEY et al[16]. The corresponding reactions are shown as follows:
TiO+C+2C12=TiC14+CO (10)
2TiO+2C12=TiC14+TiO2 (11)
Ti3O5+5C+6C12=3TiC14+5CO (12)
2Ti3O5+2C12=TiC14+5TiO2 (13)
The reactions indicate that carbochlorination of low-valent titanium oxide led to the increase of REq.
5 Experimental results and discussion
Carbon/slag molar ratio (n(C)?n(TiO2)) can be adjusted by change of petrocoke mass fraction (slag/coke) in solid mixture whereas amount of chlorine (n(Cl2)?n(TiO2)) can be adjusted by change of the gas apparent velocity of chlorine (vg) and amount of solid mixture at the inlet (Gs) to optimize experimental effect. The experiment was herein carried out under technological conditions of reaction temperature range of 973-1 073 K, vg=0.9 m/s, Gs=5.8 kg/h and mass ratio of slag to coke of 100?30, which was corresponding to the initial material molar ratio n(C)?n(Cl2)?n(TiO2)= 2.2?2.0?1.0.
WANG and MA[9] compared REq with CO/CO2 molar ratio (RRe) of off-gas produced respectively by technological processes with fluidized bed, molten salt chlorination and shaft furnace with the assistance of some references. The comparison shows that RRe of shaft furnace is the datum most close to REq; RRe of molten salt chlorination is lower and RRe of fluidized bed is the minimum. In view of the analysis mentioned above, it is predicted that REx corresponding to carbochlorination of titanium slag with novel combined fluidized bed should be different from REq.
The conversion of TiO2 (x(TiO2)) increased with increasing REx, as shown in Fig.5(a), which remains increasing with increasing temperature in the range of 973-1 073 K. Therefore, REx can be also used as a reference for evaluating x(TiO2).
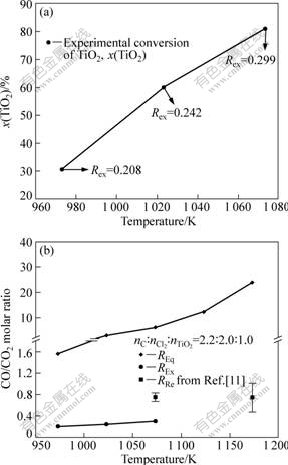
Fig.5 Analysis results of REx: (a) relationship of conversion of TiO2 and REx; (b) Comparison of REx, RRe and REq
Comparison of REx, RRe and REq in the temperature range of 973-1 173 K is also shown in Fig.5(b). REx is consistent with RRe (0.5-1.2) with reference to YAGI and OKUDAIRA[11] but less than REq.
The analysis of appearances and components of furnace slag is helpful for discovering what results in the difference between REx and REq. The polished furnace slag sample facilitated the observation and analysis of cross-sectional appearance of furnace slag as shown in Fig.6.
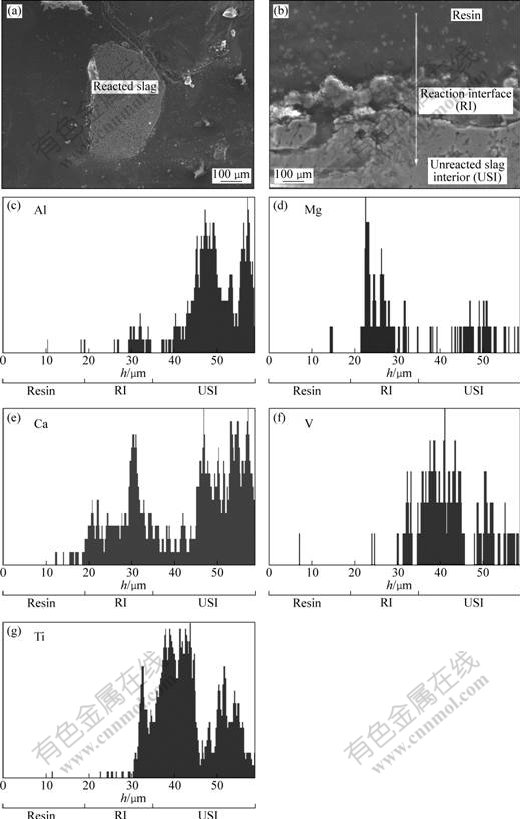
Fig.6 Morphologies and compositions analysis of furnace slag formed in nonequilibrium state of carbon- chlorination: (a) SEM image of profile of furnace slag; (b) SEM image of reaction interface; (c), (d), (e), (f) and (g) EDS linear scanning diagrams
The reaction interface (RI) can be observed clearly and the content of titanium in unreacted slag interior (USI) is high. The results can be mainly due to short retention time (about 1 s) of starting materials in combined fluidized bed, which results in incomplete reaction and non-equilibrium state for carbochlorination of oxide contained in titanium slag and further leads to the variance of REx with REq of off-gas.
Besides, Figs.6(c)-(g) show that the elements, including Ca and Mg, were enriched in the surface area (i.e. reaction interface) of furnace slag where the content of element Ti is low. The results indicate that TiCl4 produced by carbochlorination of TiO2 on the surface of titanium slag evaporated up due to its low boiling point (409.55 K at 1.013 25×105 Pa) and left less in reaction interface. In view of the reaction priority of MgO and CaO to TiO2, the non-volatile substance containing enriched Ca and Mg in the surface area can be identified as the carbochlorination products of oxide impurities (i.e., MgCl2, boiling point 1873.15 K at 1.013 25×105 Pa) and CaCl2 (boiling point 1685.15 K at 1.013 25×105 Pa)), which existed as sticky liquid and left in surface area at about 1173 K. It is worthwhile to mention that the molar ratio of CO to CO2 of product gas for the carbochlorination of oxide impurities, including CaO and MgO, is different from that for carbochlorination of TiO2 under the same technological conditions and it would prevent REx from getting access to REq in some degree. Therefore, carbochlorination of oxide impurities in titanium slag may be another reason for the variance of REx with REq of off-gas.
6 Conclusions
1) The calculated results indicate that equilibrium molar ratio of CO to CO2 increased with increasing carbon/slag molar ratio and reaction temperature but decreased with increasing chlorine amount. At the same time, the carbochlorination experiment for titanium slag was processed using novel combined fluidized bed as chlorinator and experimental molar ratio of CO to CO2 of off-gas was obtained.
2) The comparison of experimental, equilibrium and referenced molar ratios shows that REx (0.2-0.3) is nearly equal to RRe (0.5-1.2) but less than REq (≥4.3).
3) Based on the analysis of appearances and components of furnace slag, the difference between REx and REq was mainly due to short retention time (about 1 s) of starting materials in combined fluidized bed and carbochlorination of oxide impurities in titanium slag.
References
[1] YUAN Z F, WANG X Q, XU C. A new process for comprehensive utilization of complex titania ore [J]. Minerals Engineering, 2006, 19(9): 975-978.
[2] LI J, ZHU Y, MA B. Study on the thermodynamics of oxidation of TiCl4 and AlCl3 in the chloride process for titanium white pigment [J]. Engineering Chemical Metallurgy, 1993, 14(4): 305-310.
[3] YUAN Z F, ZHOU J C, LIAO D H. Carburization and desulphurisation of the semi-steel during plasma heating [J]. Steel Research, 2002, 73(5): 175-179.
[4] MO Wei, DENG Guo-zhu, LUO Fang-cheng. Titanium metallurgy [M]. Beijing: Metallurgical Industry Press, 2006: 198-200. (in Chinese)
[5] YANG F, HLAVACEK V. Effect extraction of titanium from rutile by a low-temperature chloride process [J]. Reactors, Kinetics and Catalysis, 2000, 46(2): 355-366.
[6] XU Cong, YUAN Zhang-fu, XIAO Wen-ming. One-dimensional modeling of multiple-unit pneumatic transport reactor for producing titanium tetrachloride—Ⅰ. Mathematical model [J]. The Chinese Journal of Process Engineering, 2004, 4(6): 481-499. (in Chinese)
[7] XU Cong, YUAN Zhong-fu, WANG Xiao-qiang. One-dimensional model of a multiple-unit pneumatic transport reactor for producing titanium tetrachloride—Ⅱ. Simulated results on reactor behavior [J]. The Chinese Journal of Process Engineering, 2005, 5(1): 18-22. (in Chinese)
[8] XU C, YUAN Z F, WANG X Q. Preparation of TiCl4 with the titanium slag containing magnesia and calcia in a combined fluidized bed [J]. Chinese Journal of Chemical Engineering, 2006, 14(3): 281-288.
[9] WANG He, MA Hui-juan. The composition of CO/CO2 of off-gas produced by titanium-rich materials and the effect on technological process [J]. Vanadium Titanium, 1993,21(6): 16-19. (in Chinese)
[10] LIN C I, LEE T J. On the chlorination of titanium dioxide–carbon pellet Ⅰ. Effects of gas flowrate, reaction temperature, pellet size and pellet forming pressure [J]. Journal of the Chinese Institute of Chemical Engineers, 1985, 16(1): 49-55.
[11] YAGI S, OKUDAIRA S. Chlorination of rutile with the fluidized furnace [J]. Titanium, 1963, 11: 4-12. (in Japanese)
[12] KUBASCHEWSKI O. Metallurgical thermochemistry [M]. 5th ed. London: Pergamon Press, 1979: 282-288.
[13] BARNER H E, SCHEURMAN R V. Handbook of thermochemical data for compounds and aqueous species [M]. New York: John Wiley & Sons Inc, 1978: 176-194.
[14] ARTHUR J R. Reactions between carbon and oxygen [J]. Transactions of the Faraday Society, 1951, 47(1): 164-178.
[15] SERYAKOV G V, BAKS S A. High-temperature vaporization and thermodynamics of the titanium oxides [J]. Russian Journal of Inorganic Chemistry, 1967, 15(12): 3-7.
[16] COLEY K S, TERRY S B, GRIEVESON P. Simultaneous reduction and carburization of ilmenite [J]. Metallurgical and Materials Transactions, 1995, 26B(6): 485-494.
Foundation item: Project(2008AA06Z1071) supported by the High-tech Research and Development Program of China; Project(20306030) supported by the National Natural Science Foundation of China
Corresponding author: YUAN Zhang-fu; Tel/Fax: +86-10-82529077; E-mail: afuyuan@coe.pku.edu.cn
DOI: 10.1016/S1003-6326(09)60109-6
(Edited by YANG Hua)


