
Improvement of corrosion resistance of AZ31 Mg alloy by
anodizing with co-precipitation of cerium oxide
Salah Abdelghany SALMAN, Ryoichi ICHINO, Masazumi OKIDO
Department of Material Science and Engineering, Nagoya University, Furo-cho, Chikusa-ku,
Nagoya, 464-8603, Japan
Received 18 June 2008; accepted 10 March 2009
Abstract:
Anodizing of AZ31 Mg alloy in NaOH solution by co-precipitation of cerium oxide was investigated. The chemical composition and phase structure of the coating film were determined via optical microscopy, SEM and XRD. The corrosion properties of the anodic film were characterized by using potentiodynamic polarization curves in 17 mmol/L NaCl and 0.1 mol/L Na2SO4 solution at 298 K. The corrosion resistance of AZ31 magnesium alloy is significantly improved by adding cerium oxide to alkaline solution. In addition, the surface properties are enhanced and the film contains no crack.
Key words:
anodizing; AZ31 Mg alloy; corrosion; alkaline solution;
1 Introduction
The importance of Mg alloys in various industries has increased significantly due to their high specific strength, high dimensional stability, good machinability, high castability and ability to be recycled [1-3]. These major advantages have made Mg alloys attractive in a wide range of several industrial applications where structures with light mass and high strength are required.
Unfortunately, magnesium has also some inadequate characteristics delaying its wide scale use in many applications. One of the main problems in the use of magnesium for outdoor applications is its poor corrosion resistance due to high chemical and electrochemical activity compared with other metals such as aluminum. There are two primary reasons for the poor corrosion resistance of magnesium alloys. First is the internal galvanic corrosion by second phases or impurities and second, the hydroxide film on magnesium much less stable than passive films on metals such as aluminum alloys and stainless steel[4].
Further surface treatment is needed in order to achieve good corrosion resistance that is necessary for many applications. Commonly, typical surface treatment is the chemical conversion in which chromate bath is traditionally applied in spite of being not friendly to the environment[5].
In the previous works, we investigated the effect of Ca(OH)2 and Al(NO3)3 additives to cerium-based chemical conversion solution on the morphology and corrosion resistance of the conversion layer[6].
In contrast to chemical conversion, anodizing can produce a relatively thick, dense, hard, adherent, abrasion-resistant and durable film to improve one or more surface properties, including chemical, mechanical, electrical or optical properties[7].
Anodizing of AZ31 magnesium alloy in alkaline solution with addition of Na2B4O7 and Na2SiO3 enhances the anti-corrosion property and the surface morphology [8]. It was also reported that the CeO2 treatment can be used to improve anticorrosion for alloy AZ91 in NaCl solution[9]. The coating based on CeO2 can be tailored to produce a functional gradient coating that will provide covalent bonding for strong coating adhesion and act as a barrier coating to limit the transport of water to the surface of the alloy[10]. The purpose of this work is to investigate the anodizing of AZ31 magnesium alloy in NaOH solution by co-precipitation of CeO2.
2 Experimental
2.1 Specimen
Commercially available AZ31 Mg alloy (3% Al and 1% Zn in mass fraction) was used as the substrate. After the surface of the alloy was polished up to the 2000# emery paper followed by 0.05 ?m alumina powder, the specimens were carefully cleaned with water, rinsed with acetone and dried under air. The specimens were mounted using PTFE resin tape, leaving 1 cm2 surface area.
2.2 Anodizing
Electrochemical measurements were carried out using a conventionally electrochemical cell equipped with three electrodes. Mg alloy specimen, platinum and Ag/AgCl saturated KCl were served as working, counter and reference electrode, respectively. Measurements were taken at 298 K in 1 mol/L NaOH solution with and without 1 g/L CeO2. To maintain a uniform distribution of the particles in the solution, the solution was stirred by a magnetic stirrer. The Mg alloys were anodized at a constant potential of 10 V for 30 min. After the treatment, the specimens were carefully rinsed using distilled water and dried under air before analysis.
2.3 Evaluation of corrosion property
The potentiodynamic polarization tests were carried out using a Solartron 1285 Potentiostate from Solartron Analytical, Farnborough, United Kingdom, controlled by CorrWare software from Scribner Associates Inc. The anodic polarization curves were measured in 17 mmol/L NaCl and 0.1 mol/L Na2SO4 solution, respectively, at 298 K with a scanning rate of 1 mV/s.
2.4 Morphology and structure of anodic film
The morphology and microstructure of the anodic films were observed with Hitachi S-800 scanning electron microscope(SEM) and optical microscope. The crystallography was identified with X-ray powder diffractometer(XRD).
3 Results and discussion
Point of zero charge for CeO2 is pH 6 or pH 7 [11-12], so that negatively charged CeO2 particles can be produced easily in 1 mol/L NaOH solution due to high pH value. These negatively charged particles seem to co-precipitate on the surface film during the anodizing process.
Fig.1 shows the variation of current densities with time throughout the anodizing process in the solution with and without CeO2, respectively.
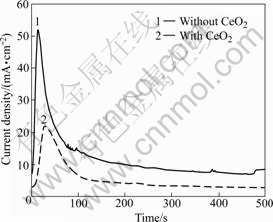
Fig.1 Current density during anodizing of AZ31 Mg alloy at 10 V in 1 mol/L NaOH without CeO2 and with CeO2
A quick rise in the current density corresponds to the dissolution reaction of Mg as shown in Eq.(1). The current density increases sharply in early time of anodizing. The highest values of the current density are20 mA/cm2 at 34 s and 53 mA/cm2 at 15 s for the solutions with and without CeO2, respectively. Mg2+ accumulates on the surface and reacts with hydroxide ion to form magnesium hydroxide film (Eq.(2)):
Mg→Mg2++2e (1)
Mg2++2OH-→Mg(OH)2 (2)
This film formation will make the current density reduce. After that, the current density remains constant with time because of the stationary dissolution and film formation. The role of CeO2 addition leads to the decrease in current density.
Fig.2 shows the morphologies of the anodized film. It can be seen that the film formed in the solution without CeO2 is of porous structure and many cracks are observed.
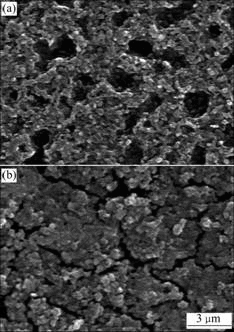
Fig.2 SEM images of surface morphology of AZ31 Mg alloy after treatment in 1 mol/L NaOH + 1 g/L CeO2 (a) and in 1 mol/L NaOH (b)
On the other hand, the film formed in the solution with CeO2 contains no crack; however, the surface is of porous structure. As CeO2 peaks were detected by XRD in Fig.3, co-precipitation of CeO2 is confirmed.
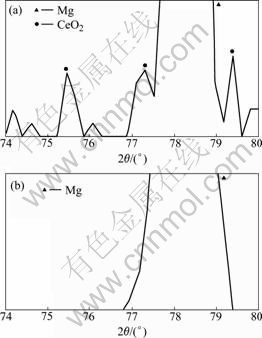
Fig.3 XRD patterns of film anodized in 1 mol/L NaOH+1 g/L CeO2 (a) and 1 mol/L NaOH (b)
Fig.4 shows the variation of open circuit potential (OCP) with time for anodized specimens in 17 mmol/L NaCl and 0.1 mol/L Na2SO4 solution at 298 K. The value of OCP for the film containing CeO2 shows more positive than that for the film without CeO2. This indicates the better passivation property of the film with CeO2.
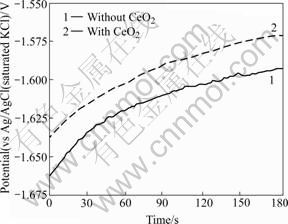
Fig.4 OCP changes for anodized films with CeO2 and without CeO2
The mechanism for the precipitation of CeO2 particles is not well understood. However, we suggest that the deposition is done by two methods.
The cerium oxide is electrophoretically deposited during anodizing due to negative charge of cerium oxide particles at high pH value of the solution.
The second suggestion was explained by SCHOLES et al[13]; a little amount of Ce(Ⅳ) ions in alkaline solution react near the metal surface according to Eq.(3):
Ce(OH)22+(aq)+2OH-(aq)→Ce(OH)4(s)→
CeO2(s)+2H2O (3)
Ce(OH)22+ from Eq.(3) is precipitated as insoluble CeO2 due to high pH value of the solution.
The corrosive behavior of the surface films was examined to characterize the corrosion resistance of the anodized films. The pitting potential of the specimens anodized in the solution with CeO2 shows the most noble compared with the others as shown in Fig.5.
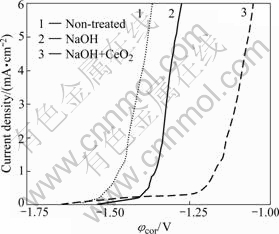
Fig.5 Anodic polarization curves before anodizing, anodizing in 1 mol/L NaOH and 1 mol/L NaOH+1 g/L CeO2
In order to evaluate the corrosion protection provided by anodic film, φcor is often used to characterize the corrosion protection of film. φcor values of non- treated, treated in NaOH, and NaOH+CeO2 specimens are -1.46, -1.35 and -1.17 V, respectively. It is clear that the film with CeO2 is much more corrosion protective than non-treated one or that produced without cerium.
4 Conclusions
1) The anodizing of AZ31 Mg alloy was performed in 1 mol/L NaOH solution with and without 1 g/L CeO2, respectively. Both anodized specimens are better than non-treated specimen in the results.
2) The surface morphology and corrosion resistance of the anodic film is enhanced with cerium oxide additive.
References
[1] KAMADO S, ASHIE T, OHSHIMA Y, KOJIMA Y. Tensile properties and formability of Mg-Li alloys grain-refined by ECAE process [J]. Mater Sci Forum, 2000, 350/351: 55-62.
[2] EMLEY E F. Principle of magnesium technology [M]. London: Pergamon Press, 1966.
[3] SALMAN S A, ICHINO R, OKIDO M. Production of alumina-rich surface film on AZ31 magnesium alloy by anodizing with co-precipitation of nano-sized alumina [J]. Materials Transaction, 2008, 49(5): 1038-1041.
[4] SONG G, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advance Engineering Materials, 1999, 1: 11-33.
[5] KIM S J, ZHOU Y, ICHINO R, OKIDO M, TANIKAWA S. Characterization of the chemical conversion films that form on Mg-Al alloys in colloidal silica solution [J]. Metals and Materials International, 2003, 9(2): 207-213.
[6] SALMAN S A, ICHINO R, OKIDO M. Development of cerium- based conversion coating on AZ31 magnesium alloy [J]. Chemistry Letters, 2007, 36(8): 1024.
[7] ZHANG Yong-jun, YAN Chuan-wei, WANG Fu-hui, LOU Han-yi, CAO Chu-nan. Study on the environmentally friendly anodizing of AZ91D magnesium alloy [J]. Surface and Coatings Technology, 2002, 161: 36-43.
[8] SALMAN S A, XIA Y, ICHINO R, OKIDO M. Effect of organic and inorganic additives on anodizing of Mg alloys in alkaline solutions [C]// 56th Annual Meeting of the International Society of Electrochemistry. Busan, Korea, 2005: 4B-058-P, 720.
[9] HAMDY A S. Enhancing the corrosion resistance of magnesium alloy AZ91D in 3.5% NaCl solution by cerate conversion coatings [J]. Anti-corrosion Methods and Materials, 2006, 53(6): 367-373.
[10] HAMDY A S. Advanced nano-particles anti-corrosion ceria based sol gel coatings for aluminum alloys [J]. Materials Letters, 2006, 60(21/22): 2633-2637.
[11] COOK L M. Chemical processes in glass polishing [J]. Non-Cryst Solids, 1990, 120: 152-171.
[12] PREUCHSUDA S, KWADWO O A. Cerium oxide slurries in CMP electrophoretic mobility and adsorption investigations of ceria/silicate interaction [J]. Journal of the Electrochemical Society, 2004, 151(10): 658-662.
[13] SCHOLES F H, SOSTE C, HUGHES A E, HARDIN S G, CURTIS P R. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings [J]. Applied Surface Science, 2007, 253/254: 1770-1780.
Corresponding author: Salah Abdelghany SALMAN; E-mail: sa.salman@hotmail.com
DOI: 10.1016/S1003-6326(08)60370-2


