J. Cent. South Univ. Technol. (2011) 18: 1871-1876
DOI: 10.1007/s11771-011-0916-y![]()
Synthesis and characterization of MgSO4·5Mg(OH)2·2H2O flake powders
FU Jian-gang(符剑刚), LIANG Wei(梁威), WANG Hui(王晖), HE Zhang-xing(何章兴)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract:
Magnesium oxysulfate (MgSO4·5Mg(OH)2·2H2O) flake powders with an average diameter of 2 μm and a thickness of 0.052 μm were prepared using magnesium sulfate and sodium hydroxide as raw materials by hydrothermal synthesis process. The composition, morphology and structural features of the hydrothermal products were examined with X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The experimental results indicate that in the conditions of n(NaOH)/n(MgSO4) of 1.25, the dosage of w(Na3PO4) crystal additives of 1.0% w(MgSO4), stirring for 5 h at 180 °C, the morphology of MgSO4·5Mg(OH)2·2H2O products is flaky and laminar, which is a kind of complex magnesium single- crystal. The recycling of MgSO4 mother liquor was also investigated to make a full use of the materials and reduce disposal. The results prove that there is no adverse effect on the yield and purity of the products.
Key words:
hydrothermal synthesis; magnesium oxysulfate; flake powders; crystal additives;
1 Introduction
Natural magnesium ores and sea-lake magnesium resources in China are expected to provide a good condition for the development of magnesium industry due to their large reserves [1]. But the present state is that the resources are not fully exploited and utilized, and the limited resources have been wasted seriously [2-3]. In recent years, the development of magnesium chemical materials is becoming a new focus in inorganic material researches. Many kinds of high value-added magnesium products like magnesium hydroxide [4-7], magnesium oxysulfate, magnesium borate whisker [8], high purity magnesium oxide whiskers, and nano-magnesium oxide [9-11] were prepared in laboratory scale. Among the various products, magnesium oxysulfate compound shows the high crystallinity and aspect ratio which make it a potential retardant material for plastics resin and rubber [12-15], and more attentions have been paid since it is economically attractive and environmentally benign product.
Magnesium oxysulfate used as ?ame-retardant ?ller in composite materials requires flaky crystal or whisker with high purity crystallinity and small particle size
[7, 16]. Some researchers have reported their work on the synthesis of magnesium oxysulfate whiskers. GAO et al [17] used magnesium chloride, ammonia and magnesium sulfate as raw materials to prepare 5Mg(OH)2·MgSO4·2H2O (512MHSH) nanowhiskers and obtained a conclusion that the thermal decomposition of nano- whiskers followed a three-step scheme. YAN et al [18] investigated that ![]() ions directed the one-dimensional growth of 512MHSH during the transformation from irregular Mg(OH)2 particles to one-dimensional 512MHSH structures. LAN et al [19] developed a hydrothermal method to synthesis and characterize magnesium oxysulfate whiskers by mixing the MgSO4 and NaOH solutions at room temperature. But there are few researches on the experimental and theoretical investigation of magnesium oxysulfate flake powders.
ions directed the one-dimensional growth of 512MHSH during the transformation from irregular Mg(OH)2 particles to one-dimensional 512MHSH structures. LAN et al [19] developed a hydrothermal method to synthesis and characterize magnesium oxysulfate whiskers by mixing the MgSO4 and NaOH solutions at room temperature. But there are few researches on the experimental and theoretical investigation of magnesium oxysulfate flake powders.
In this work, the single-crystal MgSO4·5Mg(OH)2·2H2O flake powders were synthesized by hydrothermal synthesis process. X-ray powder diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and chemical analysis were employed to characterize the composition, morphology and structural features of the hydrothermal samples. Furthermore, the recycling of MgSO4 mother liquor was studied to make full use of the raw materials and reduce disposal.
2 Experimental
2.1 Preparation of MgSO4·5Mg(OH)2·2H2O flake powders
All the reagents used in this experiment were of analytical grade (Hunan Chemical Regent Factory, China). For the preparation of MgSO4·5Mg(OH)2·2H2O flake powders, a certain ratio of sodium hydroxide and magnesium sulfate solutions were mixed at room temperature. The slurry was then transferred to a 1 L high pressure reactor (Dalian High Pressure Vessel Manufacturing Industry Ltd, China) and kept stirring (100-300 r/min). The reactor was heated (5 °C/min) gradually to 140-220 °C and then maintained in isothermal condition for 1.0-9.0 h. The reagents ratio and crystal additives were systematically varied to investigate their influence on the characteristics of the hydrothermal products. After hydrothermal treatment, some white precipitates were obtained after the reactor cooled down to room temperature naturally. After filtration, the precipitates were washed with distilled water for several times and then dried in a vacuum oven at 65 °C for 12 h to obtain the products.
2.2 Analysis
The morphologies of the samples were examined with a scanning electron microscope (SEM, Model JEM-2000EX, Japan). The infrared spectrogram of the product was recorded by a Fourier transform infrared spectrometer (FTIR, Model NEXUS670, America) using KBr pellets. X-ray powder diffraction (XRD, Model X Pert MPD, Japan) pattern of the sample was obtained using nickel-filtered Cu Kα radiation. The concentrations of soluble Mg2+ and ![]() were analyzed by EDTA titration and Ba2+ precipitation methods, respectively. OH– was determined by titrating the excess H+ with standard NaOH solution and H2O was determined by difference. The impurities of the products were determined by atomic absorption spectroscopy and the purity was determined by difference.
were analyzed by EDTA titration and Ba2+ precipitation methods, respectively. OH– was determined by titrating the excess H+ with standard NaOH solution and H2O was determined by difference. The impurities of the products were determined by atomic absorption spectroscopy and the purity was determined by difference.
3 Results and discussion
3.1 Influence of process conditions on formation of MgSO4·5Mg(OH)2·2H2O
From the viewpoint of thermodynamics, the crystal growth process is mainly determined by its intrinsic characteristics. From the viewpoint of kinetics, the growth process is on the other hand affected strongly by the external growth conditions [18, 20]. Therefore, the obtained morphology is synergistically determined by intrinsic characteristics and detailed growth conditions. Several important factors such as reagents ratio, stirring rate, reaction time and temperature are discussed here with respect to the variation of growth morphology of magnesium oxysulfate.
3.1.1 Influence of reagents ratio on formation of products
The varied reagent ratios of the reactants have great in?uence on crystal morphology and product yield. Figures 1 and 2 show the influence of the reagent ratios of the reactants on the yield and morphology of the hydrothermal products. The products have formed at 180 °C for 5 h under the static condition using the slurries synthesized by mixing NaOH and MgSO4 solutions at room temperature. When the n(NaOH)/ n(MgSO4) varies from 0.3 to 1.2, the pH of the slurry is adjusted to alkaline from acid. It is found that the OH- concentration is one of the critical factors for the formation of the products, and the hydrothermal synthesis easily happens in weak alkaline condition [13]. The yield of the products increases along with the ratio of n(NaOH)/n(MgSO4) increasing to 1.25. Therefore, the flake products composed of magnesium oxysulfate are formed at the n(NaOH)/n(MgSO4) of 1.25 and 1.5 (Figs.2(b) and (c)), while the granular products are detected at the n(NaOH)/n(MgSO4) of 2.0 (Fig.2(a)), indicating that the over-added NaOH solution has an adverse affect on the stable existence of magnesium oxysulfate products.
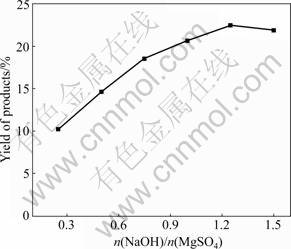
Fig.1 Influence of different reagents ratios on yield of products

Fig.2 SEM images of hydrothermal products obtained at different n(NaOH)/n(MgSO4) ratios (Temperature: 180 °C, time: 5 h, stirring rate: 200 r/min): (a) 2.00; (b) 1.50; (c) 1.25
3.1.2 Influence of stirring rate on morphology of products
The Mg(OH)2 intermediate product can be synthesized by mixing NaOH and MgSO4 solutions at room temperature, and probably interferes the mass transfer of the system due to its high viscidity. In addition, the hydrothermal reaction is a solid-liquid reaction. It is important to stir the mixture to ensure sufficient solid-liquid contact and avoid the forming of aegagroupilus in insufficient mixing process. Figure 3 shows the SEM images of the hydrothermal products prepared under different stirring rates (100-300 r/min). The morphologies of the products indicate that the growth of crystal is affected by the varied stirring rates of hydrothermal reaction. And the quick formation of the precipitates occurs at stirring rate of 300 r/min, which is unfavorable for the growth of crystals, leading to the formation of the granular products (Fig.3(b)). In contrary, the morphologies of the products formed at low agitation (R=100 r/min) are flaky with poor dispersion and low diameter-to-thickness ratio. Figure 3(c) shows that the morphology of the products formed at stirring rate of 200 r/min has high diameter-to-thickness ratio. It is proved that proper stirring is essential in synthesis process.
3.1.3 Influence of temperature on morphology of products
Figure 4 shows the SEM images of the hydrothermal products formed at varied temperatures (140-220 °C) using the slurries synthesized by mixing NaOH and MgSO4 solutions at room temperature. The hydrothermal reaction temperature mainly in?uences the concentration of hydroxyl. When the hydrothermal temperature decreases, the OH- concentration in aqueous solution increases [21], which suspends the dissolution of Mg(OH)2 and restrains the growth of flake products (Fig.4(a)). Working at low temperature favors a slow nucleation process and growth of the crystallites, which leads to larger but fewer particles. At 220 °C, the OH- concentration in aqueous solution decreases, the thermal decomposition of magnesium oxysulfate may occur, and this process is a dissolution of amorphous Mg(OH)2 and recrystallization of Mg(OH)2 and magnesium oxysulfate.
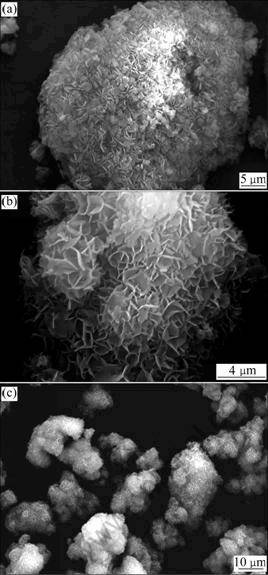
Fig.3 SEM images of hydrothermal products obtained at different stirring rate (Temperature: 180 °C, time: 5 h, n(NaOH)/n(MgSO4): 1.25): (a) R=100 r/min; (b) R=200 r/min; (c) R=300 r/min
3.1.4 Influence of reaction time on formation of products
In inorganic hydrothermal synthesis process, reaction time has some influence on the composition, appearance and yield of the products. Figure 5 shows the influence of reaction time on the yield of the hydrothermal products. The yield of MgSO4· 5Mg(OH)2·2H2O increases along with the reaction time. The induction period of crystal nucleation occurs at the beginning of the synthesis process, and the crystal growth rate is rapid when the nucleation conditions are obtained. And when the reaction time prolongs to 5 h, the yield keeps basically stable.
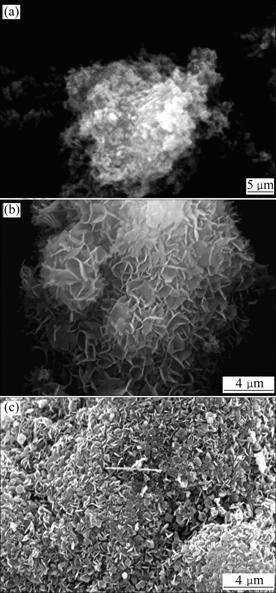
Fig.4 SEM images of hydrothermal products formed at varied temperatures (140-220 °C) (Stirring rate: 200 r/min, time: 5 h, n(NaOH)/n(MgSO4):1.25): (a) T=140 °C; (b) T=180 °C; (c) T= 220 °C
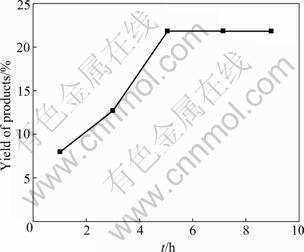
Fig.5 Influence of reaction time on yield of products (Temperature: 180 °C; Stirring time: 200 r/min; n(NaOH)/ n(MgSO4)=1.25)
3.1.5 Influence of crystal additives on morphology of products
The growth habit of crystals is often modi?ed by their growing environment. For example, the habit may be modi?ed by the presence of crystal additives or impurities [22], which bind to the growth sites and reduce the rate of attachment of solute molecules. For the improvement of magnesium oxysulfate crystallization, NaH2PO4 and Na3PO4 were utilized as crystal modi?ers in the hydrothermal synthesis process. Figure 6 shows the morphologies of the hydrothermal products synthesized by adding different crystal additives to the hydrothermal reactor. When NaH2PO4 and Na3PO4 were added to the solution, a large quantity of flake products are obtained (Figs.6(a) and (b)). These flake powers are about 2.0 μm in diameter and 0.052 μm in thickness. Compared with those without crystal additives (Figs.2(c)), the crystallization of the products is more complete with the crystal packing modified.
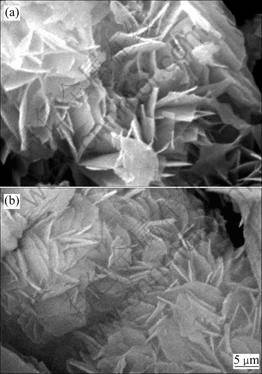
Fig.6 SEM images of hydrothermal products synthesized by adding different crystal additives (Temperature: 180 °C, stirring rate: 200 r/min; time: 5 h; n(NaOH)/n(MgSO4): 1.25): (a) w(NaH2PO4)=1.0%; (b) w(Na3PO4)=1.0%
3.1.6 Influence of recycling of MgSO4 mother liquor on yield and purity of products
The yield of magnesium oxysulfate is only about 24% due to the limited NaOH amount in the hydrothermal synthesis process, and parts of MgSO4 are still dissolved in the solution. Therefore, the recycling of MgSO4 mother liquor was investigated to make a full use of the raw materials and reduce disposal. Table 1 lists the yield and purity of the magnesium oxysulfate products by recycling the MgSO4 mother liquor. The results show that the yield of the products increases and the purity is still 99.8% even when the MgSO4 mother liquor has been recycled twice. This suggests that the recycling of MgSO4 mother liquor has no adverse effect on the yield and purity of the products.
Table 1 Influence of recycling of MgSO4 mother liquor on yield and purity of products

3.2 X-ray diffraction pattern of products
Figure 7 shows the XRD pattern of the as-prepared sample synthesized at 180 °C for 5 h under the static conditions mentioned above. The main diffraction peaks are at d=0.682 5 (I/I0=59), 0.511 6 (I/I0=100), 0.388 7 (I/I0=68), 0.388 1 (I/I0=36), 0.259 9 (I/I0=33), 0.225 3 (I/I0=57), 0.201 0 (I/I0=21) and 0.198 9 nm (I/I0=34), which can be indexed with respect to the orthorhombic structure of MgSO4·5Mg(OH)2·2H2O with lattice parameters a=0.717 7(1) nm, b=0.980 4(2) nm, c= 1.277 5(2) nm, V=0.898 9(2) nm-3, Z=4 and Dc=2.476 g/cm3. The XRD pattern indicates that the sample consists of pure phase of MgSO4·5Mg(OH)2·2H2O.
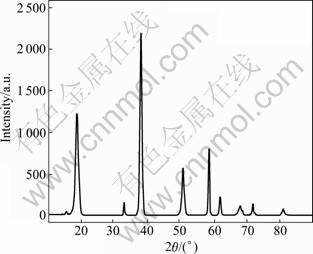
Fig.7 XRD pattern of as-prepared sample (Temperature: 180 °C; stirring rate: 200 r/min; time: 5 h, n(NaOH)/n(MgSO4): 1.25, w(Na3PO4): 1.0%)
3.3 FTIR spectrogram of products
Figure 8 presents the infrared spectrogram of MgSO4·5Mg(OH)2·2H2O in the region 4 000-400 cm-1. The FTIR spectrum shows that a strong absorption band occurs in the range 3 000-3 700 cm-1 due to the OH- vibrations originating from both lattice and coordinate water molecules. This broad band also indicates the presence of strong hydrogen bonding. The sharp absorption band at 1 654.86 cm-1 is attributed to O—H bond bending vibration absorption, while those at 1 117.50 and 592.66 cm-1 are attributed to the ![]() stretching vibration.
stretching vibration.
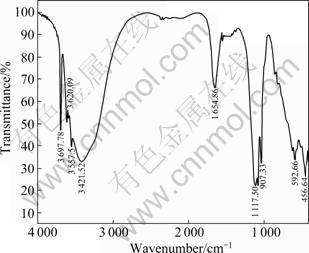
Fig.8 FTIR spectrogram of MgSO4·5Mg(OH)2·2H2O
4 Conclusions
1) MgSO4·5Mg(OH)2·2H2O flake powders are synthesized by hydrothermal method under the condition as follows: n(NaOH)/n(MgSO4) of 1.25, the dosage of w(Na3PO4) crystal additives of 1.0% w(MgSO4), stirring 5 h at 180 °C.
2) The morphologies of hydrothermal products have a close relationship with the reagents ratio, the stirring rate, reaction time and the hydrothermal temperature. After hydrothermal treatment of the slurry by mixing NaOH and MgSO4 solutions at room temperature, MgSO4·5Mg(OH)2·2H2O flake powders are formed with a diameter of 2 μm and a thickness of 0.052 μm.
3) The component of the products is pure MgSO4·5Mg(OH)2·2H2O with single-crystal structure. Furthermore, the recycling of MgSO4 mother liquor has no adverse effect on the yield and purity of the products.
References
[1] QUAN Yue. Magnesium production and application [M]. Beijing: Metallurgy Industry Press, 2008: 1-3. (in Chinese)
[2] WANG Zhao-min. Magnesite status and development trend of China [J]. China Non-Metallic Mining Industry Herald, 2006, 57(5): 6-23. (in Chinese)
[3] XU Hui, SU Yuan-zhi, LI Xin-hai, CHEN Qi-yuan, DENG Xin-rong. Technology of preparation for light magnesium oxide from bischofite [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1776-1781. (in Chinese)
[4] HSU J P, NACU A. Preparation of submicron-sized Mg(OH)2 particles through precipitation [J]. Colloids and Surfaces A, 2005, 262(1/2/3): 220-231.
[5] XIANG Lan, JIN Yong-cheng, JIN Yong. Hydrothermal formation of dispersive Mg(OH)2 particles in NaOH solution [J]. Trans Nonferrous Met Soc China, 2004, 14(2): 370-375.
[6] XUE Dong-feng, YAN Xiao-xing, WANG Lei. Production of speci?c Mg(OH)2 granules by modifying crystallization conditions [J]. Powder Technology, 2009, 191(1/2): 98-106.
[7] XU Hui, DENG Xin-rong. Preparation and properties of superfine Mg(OH)2 flame retardant [J]. Trans Nonferrous Met Soc China, 2006, 16(2): 488-492.
[8] K?R?K M, GIRGIN ?. Synthesis of magnesium borates using sodium borate and magnesium sulfate [J]. Journal of Non-Crystalline Solids, 2009, 355(16/17): 965-969.
[9] KUMARI L, LI W Z, VANNOY C H, LEBLANC R M, WANG D Z. Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO [J]. Ceramics International, 2009, 35(8): 3355-3364.
[10] WEI Zhong-qing, QI Hua, MA Pei-hua, BAO Ji-qing. A new route to prepare magnesium oxide whisker [J]. Inorganic Chemistry Communications, 2002, 5(2): 147-149.
[11] ALVARADO E, TORRES-MARTINEZ L M, FUENTES A F, QUINTANA P. Preparation and characterization of MgO powders obtained from different magnesium salts and the mineral dolomite [J]. Polyhedron, 2000, 19(22/23): 2345-2351.
[12] YUE Tao, ZHU Li-xia, XIA Shu-ping. Thermochemistry of 2MgSO4·MgO·3H2O [J]. Thermochimica Acta, 2005, 426(1/2): 49-52.
[13] ZHOU Zheng-zhi, DENG Yu-lin. Solution synthesis of magnesium hydroxide sulfate hydrate nanobelts using sparingly soluble carbonate salts as supersaturation control agents [J]. Journal of Colloid and Interface Science, 2007, 316: 183-188.
[14] DING Yi, ZHAO Hua-zhang, SUN Yu-gang, ZHANG Guang-tao, WU Hao, QIAN Yi-tai. Superstructured magnesium hydroxide sulfate hydrate ?bres–Photoluminescence study [J]. International Journal of Inorganic Materials, 2001, 3(2): 151-156.
[15] HENRIST C, MATHIEU J P, VOGELS C, RULMONT A, CLOOTS R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution [J]. Journal of Crystal Growth, 2003, 249(1/2): 321-330.
[16] HIMAWAN C, KRAMER H J M, WITKAMP G J. Study on the recovery of puri?ed MgSO4·7H2O crystals from industrial solution by eutectic freezing [J]. Separation and Puri?cation Technology, 2006, 50(2): 240-248.
[17] GAO Chuan-hui, LI Xian-guo, FENG Li-juan, XIANG Zhan-chang, ZHANG Da-hai. Preparation and thermal decomposition of 5Mg(OH)2·MgSO4·2H2O nanowhiskers [J]. Chemical Engineering Journal, 2009, 150(2/3): 551-554.
[18] YAN Xiao-xing, XU Dong-li, XUE Dong-feng. SO42- ions direct the one-dimensional growth of 5Mg(OH)2·MgSO4·2H2O [J]. Acta Materialia, 2007, 55(17): 5747-5757.
[19] LAN X, LIU F, LI J, JIN Y. Hydrothermal formation and characterization of magnesium oxysulfate whiskers [J]. Materials Chemistry and Physics, 2004, 87(2/3): 424-429.
[20] XU Dong-li, XUE Dong-feng. Chemical bond analysis of the crystal growth of KDP and ADP [J]. Journal of Crystal Growth, 2006, 286(1): 108-113.
[21] LI J, LAN X, JIN Y. Hydrothermal formation of magnesium oxysulfate whiskers in the presence of ethylenediaminetetraacetic acid [J]. Journal of Materials Science, 2006, 41(5): 1345-1348.
[22] WEISSBUCH I, LEISEROWITZ L, LAHAV M. Direct assignment of the absolute con?guration of molecules from crystal morphology [J]. Chirality, 2008, 20(5): 736-748.
(Edited by HE Yun-bin)
Foundation item: Project(50704036) supported by the National Natural Science Foundation of China; Project(08JJ3027) supported by the Natural Science Foundation of Hunan Province, China
Received date: 2010-10-01; Accepted date: 2010-12-16
Corresponding author: FU Jian-gang, PhD; Tel: +86-731-88836309; E-mail: fu_jiangang@126.com
Abstract: Magnesium oxysulfate (MgSO4·5Mg(OH)2·2H2O) flake powders with an average diameter of 2 μm and a thickness of 0.052 μm were prepared using magnesium sulfate and sodium hydroxide as raw materials by hydrothermal synthesis process. The composition, morphology and structural features of the hydrothermal products were examined with X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR). The experimental results indicate that in the conditions of n(NaOH)/n(MgSO4) of 1.25, the dosage of w(Na3PO4) crystal additives of 1.0% w(MgSO4), stirring for 5 h at 180 °C, the morphology of MgSO4·5Mg(OH)2·2H2O products is flaky and laminar, which is a kind of complex magnesium single- crystal. The recycling of MgSO4 mother liquor was also investigated to make a full use of the materials and reduce disposal. The results prove that there is no adverse effect on the yield and purity of the products.


