Trans. Nonferrous Met. Soc. China 24(2014) s125-s128
Effect of electrolyte on mechanical properties of AZ31B Mg alloy in electrolytic plasma processing
Byung Hyun AHN1, Jung Il SONG2, Bon Heun KOO1
1. School of Nano & Advace Material Engineering, Changwon National University, Changwon 641-773, Korea;
2. Department of Mechanical Engineering, Changwon National University, Changwon 641-773, Korea
Received 18 June 2013; accepted 10 Apr 2014
Abstract:
Coatings on Mg alloys were prepared using NaOH + Na2SiO3 as basic electrolyte containing electrolyte of Na2SiF6 or NaF. EPP treatment was carried out on AZ31 Mg alloys matrix under a hybrid voltage of AC of 200 V combined with DC of 260 V for 30 min. Structural and morphological analyses of ceramic coatings were analyzed by XRD and SEM. Wear and hardness of coatings were measured by pin-on disk test and Vickers hardness test. The coatings formed in Na2SiF6 and NaF electrolytes were mainly composed of MgO and Mg2SiO4. The measured micro-hardness of coating formed in Na2SiF6 electrolyte was found to be over HV 1100, while, coating formed in NaF electrolyte possessed micro-hardness of HV ~900. These results show that the mechanical properties of AZ31B Mg alloys can be enhanced by the proper selection of electrolyte agent.
Key words:
AZ31B Mg alloy; surface treatment; electrolytic plasma processing;
1 Introduction
Magnesium and its alloys are widely used in marine, aerospace, automobile and communication industries since they have high specific strength, high thermal conductivity and easily recyclable [1-5]. However, its application is limited due to its poor properties in corrosion resistance and wear resistance [6,7]. To overcome this problem, diverse surface treatment technologies, such as organic conversion, CVD, PVD, electrolytic plasma processing (EPP) and laser surface alloying (LSA) have been studied. EPP, as an environmental friendly technique developed from anodic oxidation, has been used to do surface modifications on magnesium alloys in recent years [8,9]. By this method, the protective ceramic coatings can be fabricated on light weight materials, such as Al, Mg and Ti [10-14]. EPP method forms basically conversion coatings at high voltages in an aqueous electrolyte, which relies on repetitive local dielectric breakdown and formation of plasma, modifying the coating with the incorporation of species from the electrolyte. As a result, thick, hard and well-adhered ceramic coatings layers were prepared on Mg alloys [15,16]. EPP method depends on diverse function (substrate, intensity of applied voltage, processing time, composition and concentration of electrolyte, processing time). In this work, the effect of different electrolytes on the mechanical properties of AZ31B Mg alloy treated by EPP method was observed, and the enhancement in the mechanical properties was studied.
2 Experimental
AZ31B Mg alloy was used as experimental substrate with chemical composition of 2.81% Al, 0.94% Zn, 0.40% Mn, 0.0030% Cu, 0.00062% Ni, 0.022% Si, 0.038% Fe and balance of Mg (mass fraction). Our EPP equipment was composed of Teflon bath, cooling system, circulating pump and power supply. The stainless steel was used as cathode, and Mg alloy substrate was used as anode. The dimensions of substrate were d30 mm×H10 mm. The specimens were polished with 2000 mesh SiC papers, and then were cleaned with ethanol in ultrasonic bath for 10 min. It was dried with high pressure N2 gas after cleaning. The EPP method was carried out in NaOH (2 g/L)-Na2SiO3 (12 g/L) based electrolyte containing Na2SiF6 or NaF under the hybrid voltage (DC 260 V+AC 200 V) for 30 min. The micro-structure and composition of coatings were investigated by scanning electron microscope (SEM, JP/JSM5200) and X-ray diffractometer (X’pert MPD 3040). Mechanical properties were measured by Vickers hardness and wear-resistance test.
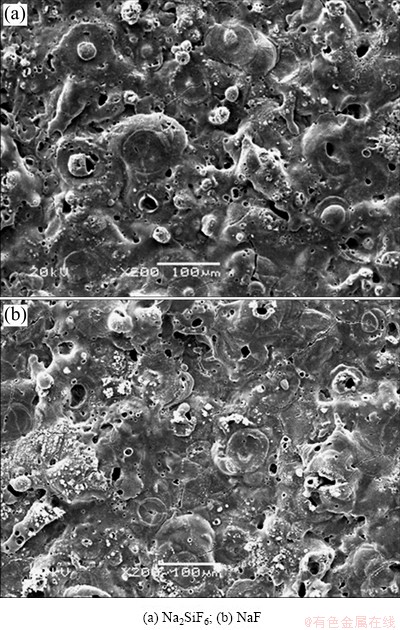
Fig. 1 SEM images of coatings formed in different electrolytes
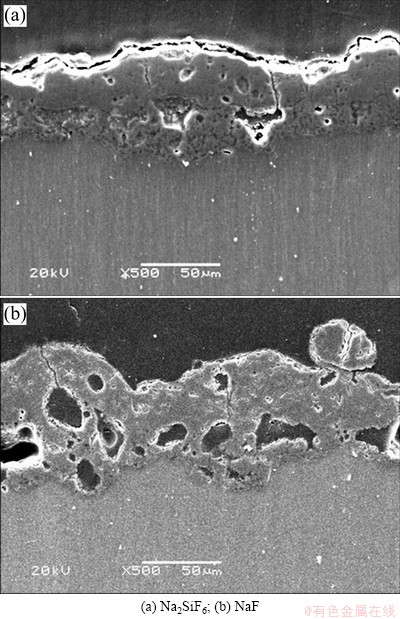
Fig. 2 Cross-sectional SEM images of coatings formed in different electrolytes
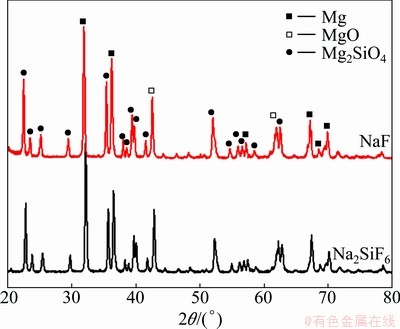
Fig. 3 XRD patterns of EPP coated AZ31B Mg alloy in Na2SiF6 and NaF electrolytes
3 Results and discussion
The surface morphology of EPP coatings formed in electrolytes including different electrolyte agents is shown in Fig. 1. Many pores are distributed all over the surface, and the specimens treated in Na2SiF6 electrolyte have more smooth surface morphology than those in NaF electrolyte. It can also be found that the pancake structure is clearly seen on the surface of specimen prepared in Na2SiF6 and NaF electrolytes. Cross sectional images, as shown in Fig. 2, depict that sample treated in Na2SiF6 electrolyte has dense coating with ~45 μm in thickness and specimen formed in NaF electrolyte has porous structure at the boundary with ~40 μm in thickness. Figure 3 shows the XRD pattern of coatings prepared in different electrolytes. The coating layer of sample treated in Na2SiF6 electrolyte is composed of MgO and Mg2SiO4. From these observations, it can be assumed that agent in electrolytes directly engages in chemical reactions [18]. Figure 4 shows the plots of intensity ratio of Mg2SiO4 to MgO as a function of electrolyte agent. It is observed from Fig. 4 that the change in intensity ratio is almost similar for both the electrolyte. The micro-Vickers hardness values of EPP-treated samples are shown in Fig. 5. The coatings formed in Na2SiF6 electrolyte have the higher value (HV ~1150) than that of the coating prepared in NaF electrolytes. The lower value of micro-hardness (HV ~300) of coatings formed in NaF electrolyte is due to the porous structure. Figure 6 shows the wear rate plots for coated and uncoated samples. Coated specimen has lower mass loss than uncoated substrate, and it can be calculated wear rate from mass loss data. The coated samples in Na2SiF6 and NaF electrolyte have low wear rate as compared with the uncoated AZ31B Mg alloy, showing the minimum wear rate for substrate coated in Na2SiF6 electrolyte. Thus, it is reasonable to state that EPP surface treatment is useful to improve mechanical properties of Mg alloys.
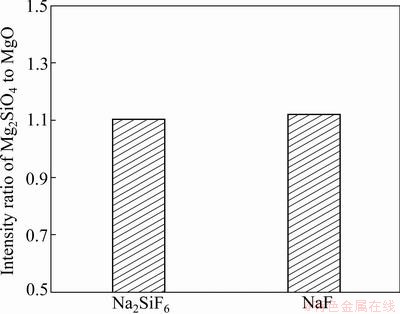
Fig. 4 Intensity ratio plot of EPP coated AZ31B Mg alloy in Na2SiF6 and NaF electrolytes
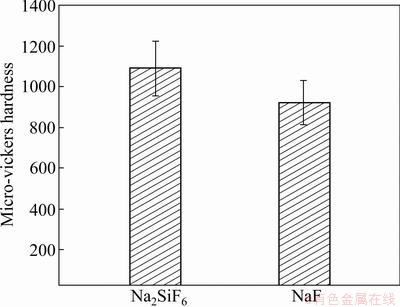
Fig. 5 Micro-hardness of coatings prepared in Na2SiF6 and NaF electrolytes
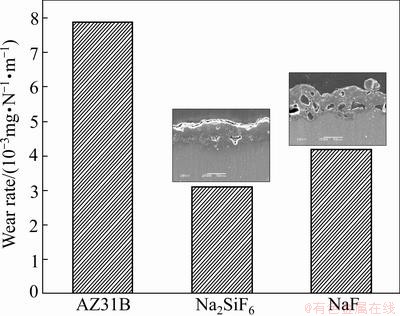
Fig. 6 Wear rate of AZ31B Mg alloy EPP treated in different electrolytes
4 Conclusions
1) AZ31B Mg alloys were treated by electrolytic plasma processing in electrolyte including Na2SiF6 and NaF electrolyte agents.
2) The coatings formed in different electrolytes have pores over the surface, while, NaF electrolyte forms coatings with more pores. The pancake structure on the surface of specimen prepared in Na2SiF6 and NaF electrolytes was observed. The coatings formed in Na2SiF6 and NaF electrolytes are composed of MgO and Mg2SiO4.
3) The micro-hardness test shows that the sample treated in Na2SiF6 electrolyte exhibits higher hardness (HV1150), while, coatings prepared in NaF electrolyte show the lower value because of porous structure. Wear test confirms that the coated specimen has lower mass loss than uncoated substrate, and coatings formed in Na2SiF6 electrolyte show superior wear resistance. These findings reveal that the mechanical properties of Mg alloys can be enhanced by choosing the suitable electrolyte agent in EPP surface treatment.
References
[1] RUDD A L, BRESLIN C B, MANSFELD F. The corrosion protection afforded by rare earth conversion coatings applied to magnesium [J]. Corros Sci, 2000, 42: 275-288.
[2] LEE B H, KIM S M, MEHTEDI M E, EVANGELISTA E, LEE C S. Effect of stress state on the high temperature workability of AZ31 Mg alloy [J]. Met Mater Int, 2001, 16: 197-203.
[3] AGHION E, BRONFIN B, ELEZER D. The role of the magnesium industry in protecting the environment [J]. Mater Proc Technol, 2001, 117: 381-385.
[4] QIU D, ZHANG M X, TAYLOR J A, KELLY P M. A new approach to designing a grain refiner for Mg casting alloys and its use in Mg–Y-based alloys [J]. Acta Mater, 2009, 57: 3052-3059.
[5] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys-A critical review [J]. J Alloys Compd, 2002, 336: 88-113.
[6] SONG G, ATRENS A. Corrosion mechanisms of magnesium alloys [J]. Advanced Engineering Materials, 1999, 1: 11-33.
[7] GHALI E. Magnesium and magnesium alloys [M]//Uhlig’s Corrosion Handbook. New York: John Wiley, 2000: 793-830.
[8] GUO H F, AN M Z, XU S, HUO H B. Formation of oxygen bubbles and its influence on current efficiency in micro-arc oxidation process of AZ91D magnesium alloy [J]. Thin Solid Films, 2005, 485: 53-58.
[9] DUAN H P, YAN C W, WANG F H. Growth process of plasma electrolytic oxidation films formed on magnesium alloy AZ91D in silicate solution [J]. Electrochim Acta, 2007, 52: 5002-5009.
[10] WU H I, CHENG Y L, LI L L, CHEN Z H, WANG H M, ZHANG Z. The anodization of ZK60 magnesium alloy in alkaline solution containing silicate and the corrosion properties of the anodized films [J]. Appl Surf Sci, 2007, 253: 9387-9394.
[11] SRINIVASAN P B, BLAWERT C, DIETZEL W. Effect of plasma electrolytic oxidation treatment on the corrosion and stress corrosion cracking behavior of AM50 magnesium alloy [J]. Mater Sci Eng A, 2008, 494: 401-406.
[12] MONFORT F, BERKANI A, MATYKINA E, SKELDON P, THOMPSON G E, HABAZAKI H, SHIMIZU K. Development of anodic coatings on aluminium under sparking conditions in silicate electrolyte [J]. Corros Sci, 2007, 49: 672-693.
[13] CURRAN J A, KALKANCI H, MAHUROVA Y, CLYNE T W. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications [J]. Surf Coat Technol, 2007, 21: 8683-8687.
[14] MATYKINA E, ARRABAL R, SKELDON P, THOMPSON G E. Transmission electron microscopy of coatings formed by plasma electrolytic oxidation of titanium [J]. Acta Biomater, 2009, 5: 1356-1366.
[15] LIANG J, GUO B G, TIAN J, LIU H W, ZHOU J F, XU T. Effect of potassium fluoride in electrolytic solution on the structure and properties of microarc oxidation coatings on magnesium alloy [J]. Appl Surf Sci, 2005, 252: 345-351.
[16] GUO H F, AN M Z. Growth of ceramic coatings on AZ91D magnesium alloys by micro-arc oxidation in aluminate–fluoride solutions and evaluation of corrosion resistance [J]. Appl Surf Sci, 2005, 246: 229-238.
电解等离子体过程中电解液对AZ31B镁合金力学性能的影响
Byung Hyun AHN1, Jung Il SONG2, Bon Heun KOO1
1. School of Nano & Advace material engineering, Changwon National University, Changwon 641-773, Korea;
2. Department of Mechanical Engineering, Changwon National University, Changwon 641-773, Korea
摘 要:以NaOH+Na2SiO3为基础电解液,分别加入Na2SiF6或NaF制备镁合金涂层。将AZ31B镁合金基体在200 V交流和260 V直流的混合电压下等离子体电解处理30 min。通过XRD和SEM方法分析陶瓷涂层的结构和形态。利用销盘式磨损实验和维氏硬度实验研究涂层的耐磨性和硬度。在Na2SiF6和NaF电解液中形成的涂层主要成分是MgO和Mg2SiO4。在Na2SiF6电解液中制备的涂层的显微硬度为HV1100,而在NaF电解液中制备的涂层的显微硬度约为HV900。结果表明:AZ31B镁合金的力学性能可以通过选择适当的电解液得到增强。
关键词:AZ31B镁合金;表面处理;等离子体电解
(Edited by Chao WANG)
Foundation item: Project (2011-0030058) supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP); Project supported by the MSIP (Ministry of Science, ICT & Future Planning), Korea; Project (NIPA-2013-H0301-13-2009) supported by the ITRC (Information technology Research Center) support program supervised by the NIPA (National IT industry Promotion Agency), Korea
Corresponding author: Bon Heun KOO; Tel: +82-55-264-5431; Fax: +82-55-262-6486; E-mail: bhkoo@changwon.ac.kr
DOI: 10.1016/S1003-6326(14)63298-2
Abstract: Coatings on Mg alloys were prepared using NaOH + Na2SiO3 as basic electrolyte containing electrolyte of Na2SiF6 or NaF. EPP treatment was carried out on AZ31 Mg alloys matrix under a hybrid voltage of AC of 200 V combined with DC of 260 V for 30 min. Structural and morphological analyses of ceramic coatings were analyzed by XRD and SEM. Wear and hardness of coatings were measured by pin-on disk test and Vickers hardness test. The coatings formed in Na2SiF6 and NaF electrolytes were mainly composed of MgO and Mg2SiO4. The measured micro-hardness of coating formed in Na2SiF6 electrolyte was found to be over HV 1100, while, coating formed in NaF electrolyte possessed micro-hardness of HV ~900. These results show that the mechanical properties of AZ31B Mg alloys can be enhanced by the proper selection of electrolyte agent.


