Trans. Nonferrous Met. Soc. China 29(2019) 2638-2645
Direct leaching of molybdenum and lead from lean wulfenite raw ore
Keng XIE, Hai-bei WANG, Sheng-dong WANG
BGRIMM Technology Group, Beijing 100160, China
Received 27 February 2019; accepted 27 September 2019
Abstract:
A direct alkaline leaching process was proposed to extract molybdenum and lead from low-grade wulfenite ore containing 2.87% Mo and 9.39% Pb. The results show that increasing temperature and alkali concentration enhances the extraction of Mo and Pb, and more than 99.7% of Mo and 64.6% of Pb are extracted under conditions of 75 °C, L/S of 2:1, leaching time of 1.0 h, initial NaOH concentration of 80 g/L and stirring speed of 100 r/min. The alkaline leaching of molybdenum follows a chemical reaction control mechanism with activation energy of 46.3 kJ/mol. Lead in the residue is recovered by hydrochloric acid leaching. 99.8% of lead is leached under the conditions of 80 °C, [MnO2]/[Pb] molar ratio of 1.3:1, sodium chloride concentration of 40 g/L, and hydrochloric acid concentration of 3 mol/L, and a product of crystallized PbCl2 with purity higher than 99.5% is obtained after cooling.
Key words:
wulfenite; molybdenum; lead; leaching; kinetics;
1 Introduction
Molybdenum is a strategic metal which has a broad range of applications and uses in the steel industry and chemical area [1-3]. Generally, molybdenum is produced from its high grade sulfide ore, molybdenite [4-9]. Besides molybdenite, wulfenite is one of the primary resources of molybdenum. Wulfenite, known as lead molybdate (PbMoO4), belongs to minerals occurring in the oxidation zones of lead-zinc deposits. Recently, researches on molybdenum extraction from wulfenite ore receive increasing concern owing to the growing market demand of molybdenum as well as the depletion of high-grade molybdenum resources.
Wulfenite deposits have been found in several world famous mining areas, e.g., Pribram in Czech, Oudida in Morocco, Sidi Renman in Algeria, Broken Hill in New South Wales of Australia, San Francisco in Sonora and Ahumada in Chihuahua of Mexico, Red Cloud Mine and Purple Passion Mine in Arizona of the United States. Considerable reserves of wulfenite ores have been found in Hunan, Yunnan, Guizhou and Shanxi Provinces in China [10]. Wulfenite is a refractory ore because the molybdenum and lead minerals are quite fine and the metal and gangue minerals are intricately associated. For a long time, wulfenite had been used merely as an ore for the smelting of lead, and the much more valuable molybdenum was lost [11]. In recent years, beneficiation-metallurgy combination process has been employed to treat wulfenite ores [12-14]. Flotation or gravity concentration of these ores produces rough concentrates containing typically 5%-15% molybdenum, but production of these concentrates is often attended by significant losses [10]. Sodium sulfide was usually used to leach molybdenum from the rough concentrates [15-17]. Mo could be leached into solution while all of Pb remained in residue, realizing an efficient separation of Mo and Pb. However, Na2S leaching was commonly carried out at a high temperature (>90 °C), resulting in high energy consumption. In addition, it often caused harmful H2S pollution.
This study aims to develop an integrated hydrometallurgical approach for direct treatment of low-grade raw wulfenite ore. From our previous mineralogy study [18], in the wulfenite ore, minerals were finely disseminated, and a lot of calcite was associated. Thus, alkaline leaching rather than acidic leaching was employed to treat the wulfenite ore. Sodium hydroxide, which appeared to be attractive for decomposition of molybdenum ores such as Ni-Mo ore [19-22], was used as the lixiviant. Experiments were carried out to investigate the process parameters such as liquid-to-solid ratio, temperature and time, initial NaOH concentration and stirring speed, to get the highest leaching yield of molybdenum. The extraction of Pb from the residue was investigated thereafter. Details of the chemistry underlying the leaching were explored.
2 Experimental
2.1 Materials
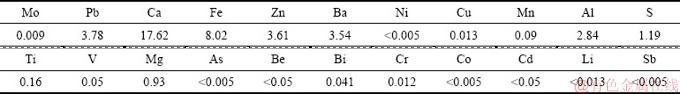
The wulfenite ore sample used in this study was obtained from a mine in Huayuan county, Hunan Province, China. The ore sample had well-cut tabular crystals of yellowish-orange color. The chemical composition of the wulfenite ore sample was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) and listed in Table 1. It contained 2.87% Mo and 9.39% Pb. Mineralogical analysis of molybdenum and lead in the sample was performed by chemical phase analysis method [18]. And the results (Table 2) show that 98.19% of Mo and 60.17% of Pb existed in wulfenite. The main mineral phases are wulfenite (PbMoO4), calcite (CaCO3), barite (BaSO4), quartz (SiO2) and galena (PbS), as characterized by an X-ray diffractometer and shown in Fig. 1.
The wulfenite ore sample was crushed and finely ground for the leaching tests. The particle size of the ground wulfenite ore sample was measured by a Malvern Mastersizer analyzer and the particle size distribution is displayed in Fig. 2. It can be seen that large amounts of wulfenite ore samples are in the range of 0.88-27.27 μm and the mean particle diameter is 6.87 μm.
Sodium hydroxide was of analytical reagent grade and used directly as received. All other chemicals used in the experiments were of analytical grade.
2.2 Experimental procedures and analysis
The wulfenite was leached using sodium hydroxide solution in a 0.5 L glass beaker. Prescribed amount of ore sample and a desired volume of sodium hydroxide solution were mixed in the beaker and then located in a thermostat water bath, where the temperature was controlled and displayed with a digital multi-meter with a precision of ±0.1 °C. The slurry was agitated with a two blade impeller driven by a variable speed motor. The stirring speed was set as required in leaching experiments. The time was recorded at the moment the machine started to run. At the end of each leaching experiment, the slurry was filtered. The resulting filter cake was thoroughly washed with hot water. The solid residues were dried to constant mass in an oven at 60 °C. Atomic absorption spectrophotometry (AAS) and inductively coupled plasma-optical emission spectrometry (ICP-OES) were used to analyze metal contents in the liquor and solid residues. The mineral phases of the residues were examined using electron microscopy and chemical phase analysis. From the leached residue, remained lead was recovered by leaching using hydrochloric acid under the similar procedures as described above.
Table 1 Chemical composition of wulfenite ore sample (wt.%)

Table 2 Phase constitution and distribution of molybdenum and lead
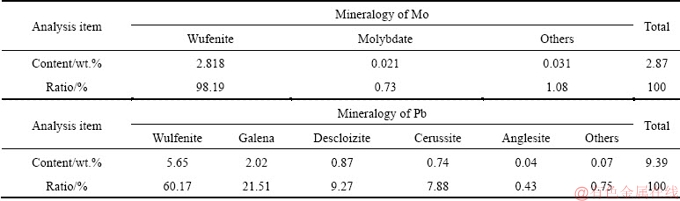
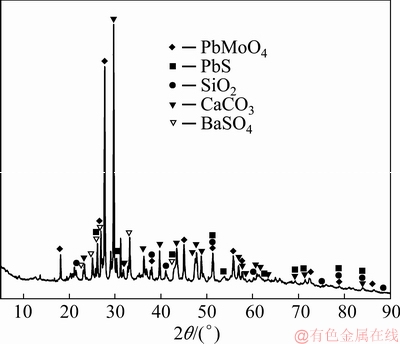
Fig. 1 X-ray diffraction pattern of wulfenite ore sample
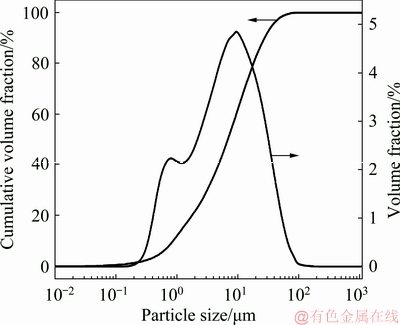
Fig. 2 Particle size distribution of ground wulfenite ore sample
3 Results and discussion
3.1 Effect of liquid-to-solid mass ratio
The effect of liquid-to-solid mass ratio (L/S) on the leaching of Mo and Pb is shown in Fig. 3. The NaOH amount is fixed at 12 g per 100 g ore. It can be seen from Fig. 3 that the leaching yields of Mo and Pb gradually increase with an increase of L/S from 1:1 to 2:1 where they reach peak values. It can be interpreted that a higher L/S means a larger liquor volume, which is favorable to diffusion process such as lixiviant NaOH transferring from solution to the surface of wulfenite and Mo and Pb from wulfenite to solution, making the leaching reaction proceed in a more throughout way. The predominated chemical reactions [23] of the leaching were assumed to occur:
PbMoO4+2OH-=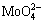 +Pb(OH)2 (1)
+Pb(OH)2 (1)
Pb(OH)2+OH-=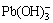 (2)
(2)
According to the above equations, Mo and Pb present in the wulfenite react with hydroxyl (OH-) to form molybdate ions 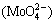 and lead-hydroxyl complexes (mainly
and lead-hydroxyl complexes (mainly 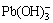 ), respectively, and were leached into solution. A higher L/S improves contact between alkali and wulfenite particles and facilitates the dissolution of Mo and Pb.
), respectively, and were leached into solution. A higher L/S improves contact between alkali and wulfenite particles and facilitates the dissolution of Mo and Pb.
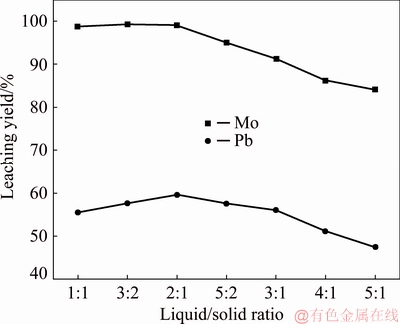
Fig. 3 Effect of liquid-to-solid mass ratio on leaching of wulfenite under conditions of NaOH amount of 12 g per 100 g ore, leaching temperature of 65 °C, stirring speed of 300 r/min, and leaching time of 1 h
However, a higher L/S under constant NaOH amount also means a lower NaOH concentration, which decreases the extraction of Mo and Pb. From Fig. 3, the leaching yield of Mo decreases from 99.01% to 84.14% and the leaching yield of Pb decreases from 59.62% to 47.44% when the L/S increased from 2:1 to 5:1. Additionally, a higher L/S reduces the Mo and Pb concentrations in the resultant leach liquor. Thus, there is a tradeoff between liquor volume and lixiviant NaOH concentration and the optimal L/S is observed to be 2:1.
3.2 Effect of leaching temperature and time
The temperature variation studies were carried out from 15 °C to elevated temperature (95 °C) with initial NaOH concentration of 60 g/L, L/S of 2:1 and stirring speed of 300 r/min. Figure 4(a) shows that the leaching yields of Mo and Pb increase with temperature in the range of 15-75 °C with a fixed leaching time of 1 h. 71.28% of Mo and 48.32% of Pb are leached at 15 °C while 99.66% of Mo and 59.30% of Pb are leached at 75 °C. Compared to Pb, Mo is more readily impacted by the leaching temperature. Further increase in temperature to 95 °C has no significant effect on the leaching of Mo but results in a decrement of approximate 8% in Pb leaching yield. The decrease in Pb leaching yield is possibly attributed to the instability of Pb-hydroxyl complexes and precipitation of Pb at a higher temperature above 80 °C or so.
The extraction of Mo is highly sensitive to temperature and the leaching efficiency of Mo is greatly enhanced at a higher temperature, as also shown in Fig. 4(b). The leaching yield of Mo is only 69.94% in a leaching time of 0.5 h and 73.69% in 3 h at 15 °C, whereas the leaching yield of Mo is more than 99.7% in a leaching time of 0.5 h and holds almost constant thereafter at a temperature above 75 °C. This indicates that the reaction is fast and 30 min is sufficient to achieve the maximum reaction at a temperature above 75 °C.
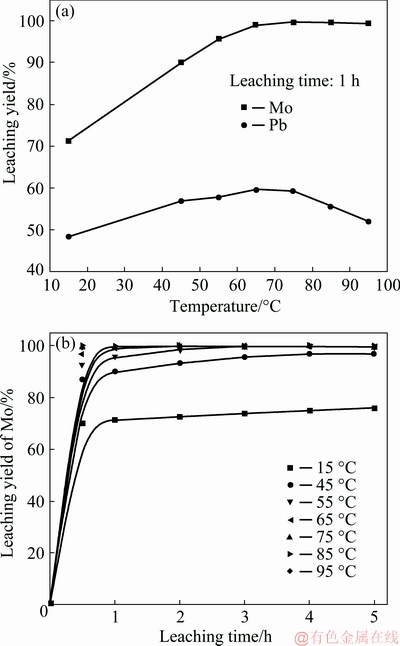
Fig. 4 Effect of temperature (a) and time (b) on leaching of wulfenite under conditions of initial NaOH solution concentration of 60 g/L, liquid-to-solid mass ratio of 2:1, and stirring speed of 300 r/min
The kinetics of leaching of Mo from wulfenite was assessed on the basis of shrinking core models. The experiment data were analyzed and fitted to different rate equations for these models [24,25], and the leaching of Mo was found to follow linear kinetics:
1-(1-η) 1/3=kst (3)
where ks is the reaction rate constant (h-1), t is reaction time elapsed (h), and η is the fraction of molybdenum dissolved.
The plots of 1-(1-η)1/3 versus leaching time at different temperatures (15, 45 and 55 °C) for leaching molybdenum are presented in Fig. 5. It can be seen from Fig. 5 that all of them give good linear fit with coefficient R2 values higher than 0.98, suggesting that the reaction of alkaline leaching of Mo from wulfenite is surface chemically controlled.
Using the rate constants determined from these curves, an Arrhenius plot, which illustrates the dependence of rate constant upon temperature, is given in Fig. 6. From Fig. 6, an Arrhenius activation energy of 46.3 kJ/ mol is calculated for the alkaline leaching of Mo from wulfenite ore. The activation energy is more than 40 kJ/mol, confirming a chemical reaction control mechanism as the rate-controlling step.
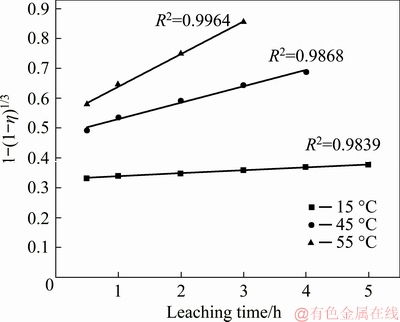
Fig. 5 Plots of 1-(1-η)1/3 vs leaching time at different temperatures for Mo leaching under conditions of initial NaOH solution concentration of 60 g/L, liquid-to-solid mass ratio of 2:1, and stirring speed of 300 r/min
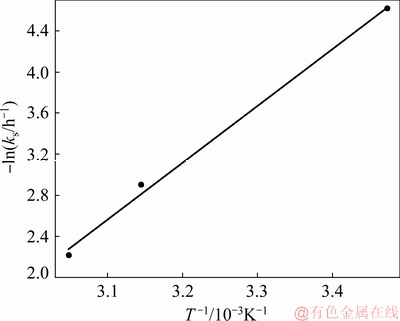
Fig. 6 Arrhenius plot for alkaline leaching of Mo from wulfenite ore
3.3 Effect of initial NaOH concentration
The effect of the initial concentration of NaOH solution on the leaching of Mo and Pb was studied by varying NaOH concentrations over the 30-100 g/L range. Figure 7 shows that the leaching yields of Mo and Pb increase significantly with the initial concentration of NaOH when the initial NaOH concentration is less than 60 g/L. 50.41% of Mo is leached together with 25.82% of Pb for [NaOH]=30 g/L while 99.66% of Mo is leached together with 59.30% of Pb for [NaOH]=60 g/L. A higher NaOH concentration correspondingly increases the alkaline amount and leads to a higher leaching rate. However, the leaching yield of Mo changes little and the leaching yield of Pb increases slowly when the initial concentration of sodium hydroxide is above 60 g/L. 99.78% of Mo and 61.55% of Pb are leached when the initial concentration of NaOH is 80 g/L. The behavior of Pb may be an effect of the formation and solubility of Pb-hydroxyl complexes in sodium hydroxide solutions. 65.74% of Pb is leached when the initial concentration of NaOH is increased to 100 g/L. Further increasing the NaOH concentration to 100 g/L is unfavorable because of higher alkaline consumption and more acid requirement in the subsequent neutralization step.
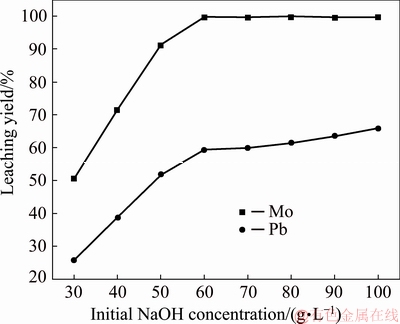
Fig. 7 Effect of initial NaOH concentration on leaching of wulfenite under conditions of leaching temperature of 75 °C, liquid-to-solid mass ratio of 2:1, stirring speed of 300 r/min, and leaching time of 1 h
3.4 Effect of stirring speed
Experiments with stirring speeds of 50-350 r/min were carried out under conditions of leaching temperature of 30 °C, L/S of 2:1, initial NaOH concentration of 80 g/L and leaching time of 1.0 h. The results are concluded in Fig. 8. It is found that the leaching yield of Mo and Pb has little dependence on the stirring speed. The leaching yields of Mo and Pb are more than 99.7% and 63.2%, respectively, and hold almost constant when a stirring speed larger than 100 r/min is employed. And the test results agree with the characteristics of chemical controlled leaching process well.
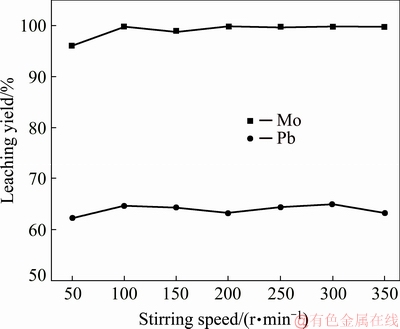
Fig. 8 Effect of stirring speed on leaching of wulfenite under conditions of leaching temperature of 75 °C, liquid-to-solid mass ratio of 2:1, leaching time of 0.5 h, and initial NaOH concentration of 80 g/L
3.5 Characterization of leach liquor and leach residue
The chemical compositions of the leach liquor and residue after leaching (under conditions of leaching temperature of 75 °C, L/S of 2:1, leaching time of 1.0 h, initial NaOH concentration of 80 g/L and stirring speed of 100 r/min) are shown in Table 3 and Table 4, respectively. 99.7% of Mo and 64.6% of Pb in the wulfenite ore are extracted and a solution containing 13.32 g/L Mo and 30.42 g/L Pb is obtained. Pb in the leachate can be recovered by precipitation with NaHS and pH adjustment with sulfuric acid. After precipitation of Pb, Mo can be separated by solvent extraction and precipitated as ammonium molybdate [26].
The leach residue is composed of 3.78% Pb, which exists mainly as galena and slightly as descloizite, as detected by electron microscopy and illustrated in Fig. 9. Chemical phase analysis further reveals that 96.33% of Pb exists in galena whereas 2.71% of Pb in descloizite.
Table 3 Chemical composition of leach liquor (g/L)
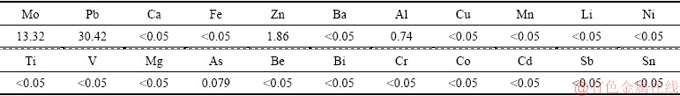
Table 4 Chemical composition of residue after alkaline leaching (wt.%)
The sizes of galena and descloizite are 5-30 μm generally, mainly in the form of monomers, a small amount of which is associated with gangue. Gangue minerals in the leach residue are mainly calcite, followed by barite and quartz.
3.6 Extraction of lead from residue by hydrochloric acid leaching
Hydrochloric acid was used to leach Pb from the leaching residue. Manganese dioxide was employed as oxidant and sodium chloride was used as a source of chloride ions [27,28]. The main reactions involved in the leaching are as follows [29-31]:
PbS+MnO2+4HCl=PbCl2+MnCl2+S+2H2O (4)
S+3MnO2+4HCl=MnSO4+2MnCl2+2H2O (5)
PbCl2+2Cl-=[PbCl4]2- (6)
Galena, manganese dioxide and hydrochloric acid react to form manganese salts, lead chloride and sulfur. Sulfur may be further oxidized to sulfate in depth, while lead chloride and chloride ions form soluble complexes [PbCl4]2- at high temperature. 99.8% of lead is leached under the conditions of leaching temperature of 80 °C, L/S of 10:1, leaching time of 1.0 h, [MnO2]/[Pb] molar ratio of 1.3:1, sodium chloride concentration of 40 g/L, hydrochloric acid concentration of 3 mol/L and stirring speed of 300 r/min. After filtration, the soluble complexes [PbCl4]2- in the solution are transformed into PbCl2 crystals after cooling [29]. As a result, lead can be recovered as precipitated lead dichloride crystals at low temperature. More than 82% of PbCl2 are crystallized when the temperature is decreased to 15 °C and held for 3 h. A product of crystallized PbCl2 with purity higher than 99.5% is obtained after washing with cold deionized water and drying.
The simplified process flowsheet, which includes the above mentioned optimum conditions, is given in Fig. 10.
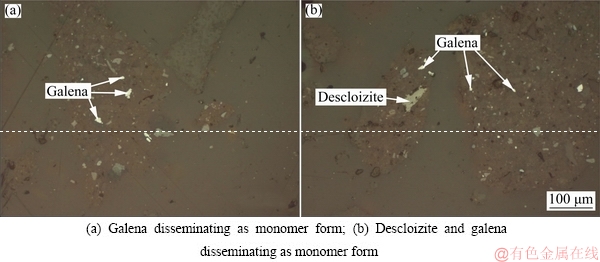
Fig. 9 Electron microscopy images of leach residue
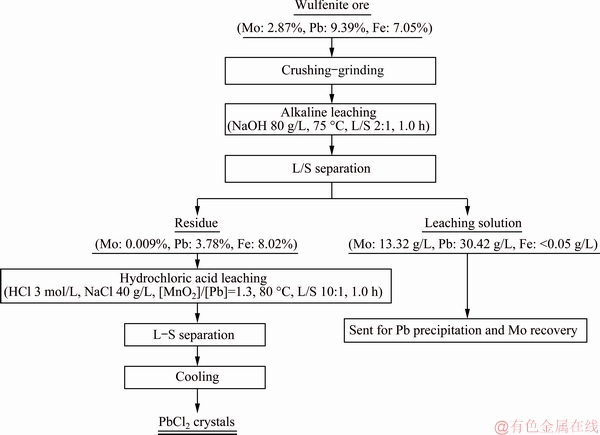
Fig. 10 Simplified process flowsheet
4 Conclusions
(1) The alkaline leaching of wulfenite ore provides a relatively simple, clean approach to extract molybdenum and lead directly from the associated gangue minerals. Dissolution behaviors of molybdenum and lead from wulfenite ore leaching are dependent on initial NaOH concentration, temperature and the liquid-to-solid mass ratio.
(2) The alkaline leaching of molybdenum from wulfenite ore follows chemical reaction control kinetics with activation energy of 46.3 kJ/mol. Increasing pH and temperature enhances the extraction of molybdenum and lead. The alkaline leaching of Mo and Pb is achieved rapidly (<1.0 h) over a temperature range of 75-95 °C. Almost all of Mo and 64.6% of Pb in the wulfenite are extracted under conditions of leaching temperature of 75 °C, L/S of 2:1, leaching time of 1.0 h, initial NaOH concentration of 80 g/L and stirring speed of 100 r/min.
(3) The remained Pb in the leach residue exists mainly as galena and slightly as descloizite, which can be completely leached by hydrochloric acid with addition of manganese dioxide and recovered as PbCl2 crystals.
References
[1] NATHAN L. Molybdenum [M]. New York: Cavendish Square Publishing, 2006: 10-40.
[2] Braithwaite E R. Occurrence, extraction, production and uses of molybdenum [C]//Braithwaite E R, Haber J. Studies in Inorganic Chemistry. Amsterdam: Elsevier, 1994: 91-93.
[3] Wang Lu, ZHANG guo-hua, DANG Jie, CHOU Kuo-chih. Oxidation of roasting of molybdenite concentrate [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4167-4174.
[4] Zhou Qiu-sheng, YUN Wei-tao, XI Jun-tao, LI Xiao-bin, QI Tian-gui, LIU Gui-hua, PENG Zhi-hong. Molybdenite-limestone oxidizing roasting followed by calcine leaching with ammonium carbonate solution [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(7): 1618-1626.
[5] Khoshnevisan A, Yoozbashizadeh H, Mozammel M, Sadrnezhaad S K. Kinetics of pressure oxidative leaching of molybdenite concentrate by nitric acid [J]. Hydrometallurgy, 2012, 111-112: 52-57.
[6] Fu Yun-feng, XIAO Qing-gui, GAO Yi-ying, NING Peng-ge, XU Hong-bin, Zhang Yi. Direct extraction of Mo(VI) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies [J]. Transactions of Nonferrous Metals Society of China, 2018, 28(8): 1660-1669.
[7] Jiang Kai-xi, Wang Hai-bei, Zou Xiao-ping, Zhang Lei, Zhang Bang-sheng, The development of China’s molybdenum metallurgical technologies [C]//Wang Shi-jie, Dutrizac J E, FREE M L, Hwang J Y, Kim D, CHEN T T. Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterization. New Jersey: John Wiley & Sons Inc, 2012: 39-50.
[8] Rajput P, Janakiram V, Jayasankar K, Angadi S, Bhoi B, MUKHERJEE P S. Environmental benign process for production of molybdenum metal from sulphide based minerals [J]. Journal of The Institution of Engineers (India): Series D, 2017, 98(2): 231-237.
[9] Xie Keng, Wang Hai-bei, Zhang Bang-sheng. Progress of pressure hydrometallurgy of molybdenite concentrate [J]. Metal Mine, 2014(1): 74-79. (in Chinese)
[10] Xie Keng, Wang Hai-bei, Wang Sheng-dong. Process and research status of molybdenum extraction from wulfenite [C]//The Proceedings of The 6th Conference on Rare Metals. Xiamen: Nonferrous Society of China, 2013: 270-279. (in Chinese)
[11] Kang Shao-hui, Meng Jin, Wang Hong-ming, Wang Ping, Yang Jian-fei. Research status of hydrometallurgical process of molybdenum minerals [J]. Hydrometallurgy of China, 2012, 31(6): 333-337. (in Chinese)
[12] Zhu Yao-ping. Benefication of certain color wulfenite [J]. Nonferrous Metals, 2010, 62(2): 74-78. (in Chinese)
[13] Chen Jian-hua, Wei Zhong-wu, Zhu Xin-yu. Experimental study on a new flotation technique for slimy wulfenite with high limonite content [J]. Mining R & D, 2007, 27(6): 37-39. (in Chinese)
[14] Zhou Xin-min, Song Xiang-yu, Gao Zhi. Research on comprehensive recovery of wulfenite polymetallic ore in Henan [J]. Mining and Metallurgical Engineering, 2011, 31(5): 56-59. (in Chinese)
[15] Ma Fei, Zhao Zhong-wei, Cao Cai-fang, Zhang Gang, Huo Guang-sheng, Chen Ai-liang, Li Hong-gui. Thermodynamic analysis on sodium sulfide decomposition of wulfenite [J], China Molybdenum Industry, 2008, 32(1): 44-47. (in Chinese)
[16] Yang Shao-wen, Yang Yao-hua, Liu Hong-zhao, Gao Zhao-guo. Research on molybdenum leaching from low grade lead- molybdenum rough concentrate [J]. Nonferrous Metals (Metallurgy Part), 2011(5): 31-33. (in Chinese)
[17] Liao Yuan-shuang, Yang Da-jin, Lu Shun-li. Study on molybdenum recovering from wulfenite [J]. Nonferrous Metals (Metallurgy Part), 2006(4): 26-27. (in Chinese)
[18] Wang Hui, Xiao Yi-wu, Wang Ming-yan, Xie Keng. A new chemical phase analysis method of Pb in a lead ore including wulfenite and descloizite [J]. Nonferrous Metals Engineering, 2015, 5(2): 39-43. (in Chinese)
[19] Wang Ming-shuang, Wei Chang, Fan Gang, Li Min-ting, Deng Zhi-gan, Wang Si-fu. Selective extraction of Mo from a Ni-Mo ore using pressure alkaline leaching [J]. Hydrometallurgy, 2015, 153: 6-11.
[20] Zhao Zhong-wei, Li Jiang-tao, Cao Cai-fang, Huo Guang-sheng, Zhang Gang, Li Hong-gui. Recovery and purification of molybdenum from Ni-Mo ore by direct air oxidation in alkaline solution [J]. Hydrometallurgy, 2010, 103(4): 68-73.
[21] Zhao Zhong-wei, Zhang Gang, Huo Guang-sheng, Li Hong-gui. Kinetics of atmospheric leaching molybdenum from metalliferous black shales by air oxidation in alkali solution [J]. Hydrometallurgy, 2009, 97(4): 233-236.
[22] Wang Ming-shuang, Wei Chang, Fan Gang, Deng Zhi-gan, Wang Si-fu, Wu Jun. Molybdenum recovery from oxygen pressure water leaching residue of Ni-Mo ore [J]. Rare Metals, 2013, 32(2): 208-212.
[23] Zhang Gang, Zhao Zhong-wei, Li Jiang-tao, Chen Ai-liang, Huo Guang-sheng, Li Hong-gui. Thermodynamics analysis on sodium hydroxide decomposition of wulfenite [J]. Journal of Central South University (Science and Technology), 2008, 39(5): 902-906. (in Chinese)
[24] Hem S R, Saradindukumar R. Kinetics of metallurgical processes [M]. Singapore: Springer Nature Singapore, 2008.
[25] CHEN Jia-yong. Hand book of hydrometallurgy [M]. Beijing: Metallurgical Industry Press, 2005. (in Chinese)
[26] JIANG Kai-xi, WANG Hai-bei, WANG Sheng-dong, XIE Keng, ZHANG Lei, JIANG Ying-ping, FENG Ya-ping, HUANG Sheng, WEI Gang, ZHAO Lei, WANG Ren-jian, LI He, LIU Wei . A clean method for treatment of wulfenite ore [P]. CN Patent: 2014102444187. 2015-08-12.
[27] Rusen A,Sunkar A S, Topkaya Y A. Zinc and lead extraction from Cinkur leach residues by using hydrometallurgical method [J]. Hydrometallurgy, 2008, 93: 45-50.
[28] Turan M D, ALTUNDOGAN h S, Tumen F. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75: 169-176.
[29] Holdich R G, Lawson G J. The solubility of aqueous lead chloride solutions [J]. Hydrometallurgy, 1987, 19(2): 199-208.
[30] LI Guang-hui, YOU Zhi-xiong, SUN Hu, SUN Rong, PENG Zhi-wei, ZHANG Yuan-bo, JIANG Tao. Separation of rhenium from lead-rich molybdenite concentrate via hydrochloric acid leaching followed by oxidative roasting [J]. Metals, 2016(6): 281-292.
[31] Zhan Xue-hui, Li Zhao-hui, Zhan Han-hui, Li Fei, Cao Fen, Li Xia, Zhou Sui-an. Leaching of galena by manganese dioxide in hydrochloric acid solution [J]. Journal of Central South University (Science and Technology), 2012, 43(6): 67-71. (in Chinese).
低品位彩钼铅矿直接浸出钼和铅
谢 铿,王海北,汪胜东
北京矿冶科技集团有限公司,北京 100160
摘 要:以含钼2.87%、铅9.39%的低品位彩钼铅矿为对象,采用直接碱浸工艺提取钼和铅。结果表明,升高温度或碱浓度有利于钼和铅的浸出,在浸出温度75 °C、液固比2:1、浸出时间1.0 h、NaOH初始浓度80 g/L、搅拌速度100 r/min的条件下,钼和铅的浸出率分别大于99.7%和64.6%。钼的碱浸受化学反应控制,反应活化能为46.3 kJ/mol。进一步采用盐酸浸出回收碱浸渣中的铅,在浸出温度80 °C、MnO2/Pb摩尔比1.3:1、氯化钠浓度40 g/L、盐酸浓度3 mol/L的条件下,铅的浸出率达到99.8%;含铅的浸出液冷却后得到氯化铅结晶,纯度可达99.5%以上。
关键词:彩钼铅矿;钼;铅;浸出;动力学
(Edited by Bing YANG)
Foundation item: Project (2012BAB10B06) supported by the National Science and Technology Pillar Program of China; Project (51434001) supported by the National Natural Science Foundation of China
Corresponding author: Keng XIE; Tel: +86-10-59069561; E-mail: xie1011@163.com
DOI: 10.1016/S1003-6326(19)65170-8
Abstract: A direct alkaline leaching process was proposed to extract molybdenum and lead from low-grade wulfenite ore containing 2.87% Mo and 9.39% Pb. The results show that increasing temperature and alkali concentration enhances the extraction of Mo and Pb, and more than 99.7% of Mo and 64.6% of Pb are extracted under conditions of 75 °C, L/S of 2:1, leaching time of 1.0 h, initial NaOH concentration of 80 g/L and stirring speed of 100 r/min. The alkaline leaching of molybdenum follows a chemical reaction control mechanism with activation energy of 46.3 kJ/mol. Lead in the residue is recovered by hydrochloric acid leaching. 99.8% of lead is leached under the conditions of 80 °C, [MnO2]/[Pb] molar ratio of 1.3:1, sodium chloride concentration of 40 g/L, and hydrochloric acid concentration of 3 mol/L, and a product of crystallized PbCl2 with purity higher than 99.5% is obtained after cooling.


