J. Cent. South Univ. Technol. (2009) 16: 0242-0246
DOI: 10.1007/s11771-009-0041-3
![]()
Recovery of copper sulfate after treating As-containing wastewater by precipitation method
ZHENG Ya-jie(郑雅杰), WANG Yong(王 勇), XIAO Fa-xin(肖发新), LUO Yuan(罗 圆)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
The solid sodium hydroxide neutralized acidic As-containing wastewater till pH value was 6. Green copper arsenite was prepared after copper sulfate was added into the neutralized wastewater when the molar ratio of Cu to As was 2?1 and pH value of the neutralized wastewater was adjusted to 8.0 by sodium hydroxide. The arsenious acid solution and red residue were produced after copper arsenite mixed with water according to the ratio of liquid to solid of 4?1 and copper arsenite was reduced by SO2 at 60 ℃ for 1 h. The white powder was gained after the arsenious acid solution was evaporated and cooled. Copper sulfate solution was obtained after the red residue was leached by H2SO4 solution under the action of air. The results show that red residue is Cu3(SO3)2?2H2O and the white powder is As2O3. The leaching rate of Cu reaches 99.00% when the leaching time is 1.5 h, molar ratio of H2SO4 to Cu is 1.70, H2SO4 concentration is 24% and the leaching temperature is 80 ℃. The direct recovery rate of copper sulfate is 79.11% and the content of CuSO4?5H2O is up to 98.33% in the product after evaporating and cooling the copper sulfate solution.
Key words:
As-containing wastewater; SO2; As2O3; copper sulfate; copper arsenite; recovery;
1 Introduction
The acidic wastewater comes from the mist of copper smelting washed by water, which contains plentiful arsenic and other metal ions, e.g. Cu2+, Fe2+, Zn2+ and Pb2+. Arsenic in the water is extremely harmful [1-5]. It is decided as the first-class carcinogenic substance by the Center of Disease Control (CDC) of the Unite State and International Cancer Institute [6]. At present, the ferrous salt flocculation process [7-8] and sodium sulfide method [9-11] are widely used to treat this As-containing wastewater in the world. The ferrous salt flocculation process results in a large amount of the flocculation residue and the secondary pollution [12-13]. The sodium sulfide method has high cost and produces a lot of arsenic sulfide residue. It is necessary to treat arsenic sulfide further. The copper sulfate replacement method was invented by the Sumitomo Company of Japan to use copper sulfate to replace arsenic sulfide and produce copper sulfide and the arsenious acid solution. Copper sulfide was separated after air oxidizes arsenious acid to H3AsO4. SO2 reduces H3AsO4 to arsenious acid. As2O3 is made by evaporating and cooling arsenious acid solution. The copper sulfate replacement method cannot be used broadly because the technique is very complicated and it is great difficulty to recover copper sulfate from copper sulfide although arsenic is recovered [14-15].
The precipitation method of copper sulfate treated As-containing wastewater to prepare As2O3 [16-20]. This method consists of copper sulfate precipitation, SO2 reduction, As2O3 preparation and copper sulfate recovery. Copper sulfate precipitates AsO2- in the wastewater to produce copper arsenite, SO2 reduces copper arsenite to produce HAsO2 solution and Cu3(SO3)2·2H2O. HAsO2 solution is evaporated to produce As2O3. H2SO4 leaches Cu3(SO3)2·2H2O under the action of air to produce copper sulfate. The cost of copper sulfate precipitation method decreases rapidly by recovering so that the copper sulfate precipitation method has a wide application in the industry to treat As-containing wastewater. The recovery of copper sulfate from Cu3(SO3)2·2H2O produced by copper sulfate precipitation method has not been reported. Therefore, the recovery of copper sulfate from Cu3(SO3)2·2H2O was studied in this work.
2 Experimental
2.1 Experimental steps
2.1.1 Preparation of copper arsenite
The impurities was removed by filtration after solid NaOH was adjusted the pH value of the wastewater to 6.0. The green copper arsenite was prepared through precipitating, filtrating, washing and drying after copper sulfate (industrial grade) was put into the neutralized wastewater and the pH value of the wastewater was adjusted to 8.0 [18].
2.1.2 Reduction of copper arsenite
Copper arsenite mixed with water according to the ratio of liquid to solid as 4?1 (mL?g). The red reduction residue and the arsenious acid solution were gained after copper arsenite was reduced by SO2. As2O3 was obtained from the arsenious acid solution by evaporating and cooling.
2.1.3 Recovery of copper sulfate
The red reduction residue was leached in sulfuric acid solution with air at a certain temperature. Copper sulfate crystal was gained from the leached solution by evaporating and cooling.
2.2 Technique worksheet
The technique worksheet is shown in Fig.1.
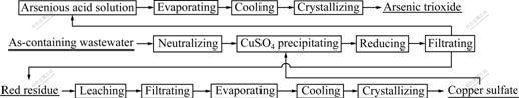
Fig.1 Worksheet of recovering copper sulfate by copper sulfate precipitation method
2.3 Analysis and detection
The arsenic content was detected by potassium bromate method. The copper content was detected by iodometric method.
The other components were detected by X-ray fluorescence (XRF) (PW2424, Philip Company of Holand). The phase of the solid product was detected by X-ray diffraction (XRD) (The emission target of XRD was Cu Kα, the emission power was 50 kV×100 mA, the step width was 0.01?, the scanning rate was 8 (?)/min and 2θ was 5?-80?, D/max-rA, Rigaku Corporation of Japan).
3 Results and discussion
3.1 Preparation of copper arsenite with As-containing wastewater
According to the reaction conditions reported in Refs.[16-17], 100 L of acidic As-containing wastewater was neutralized to adjust pH value to 6.0 by solid NaOH. The neutralized wastewater was filtrated after precipitating. The components of the As-containing wastewater are listed in Table 1. Copper sulfate was then added in the neutralized wastewater agitating for 1 h according to the molar ratio of Cu to As of 2?1. 1.626 6 kg green copper arsenite was gained by filtrating, washing and drying. The components of copper arsenite are listed in Table 2.
Table 1 Components of As-containing wastewater (mass concentration, g/L)
![]()
Table 2 Components of copper arsenite from As-containing wastewater (mass fraction, %)
![]()
As(T) is total content of As(Ⅲ) and As(Ⅴ) in the wastewater. Table 1 shows that the content of As(Ⅴ) equals 0 and the content of As(Ⅲ) is 4.500 g/L. Arsenic of the mist of copper smelting and wastewater exist in the form of As2O3 and HAsO2, respectively. Therefore the content of As(Ⅴ) equals 0.
It can be seen from Table 2 that the mass fractions of Cu and As are 46.610% and 27.390% respectively in the product copper arsenite. This means that the precipitation rates of Cu and As are 99.0% and 99.5%, respectively.
As(Ⅲ) reacts with Cu2+ after the other elements of Fe, Bi and Sb etc, in the wastewater are removed when pH value is adjusted to 6.0. The reactions are shown as follows:
Cu2++2HAsO2+2OH-=Cu(AsO2)2↓+2H2O (1)
Cu2++2OH-=Cu(OH)2↓ (2)
Copper sulfate was excessively added in order to precipitate As completely.
3.2 Reduction of copper arsenite by SO2
Copper arsenite mixed with water according to the ratio of liquid to solid as 4?1 (mL?g). SO2 gas was inflated into the slurry of copper arsenite to reduce copper arsenite at 60 ℃ for 1 h. The red residue and the arsenious acid solution were respectively gained after filtrating. The white powder was obtained after the arsenious acid solution was evaporated and cooled.
The XRD patterns of the white powder and the red residue are shown in Fig.2 and Fig.3, respectively. The components of the red reduction residue are listed in Table 3.

Fig.2 XRD pattern of As2O3
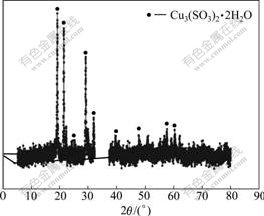
Fig.3 XRD pattern of reduction residue
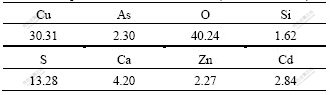
Table 3 Components of reduction residue (mass fraction, %)
It can be seen from Fig.2 that the white powder is As2O3. Arsenic trioxide separates out of evaporated arsenious acid solution by means of cooling because the concentration of arsenious acid increases after evaporating. The reaction is as follows:
2HAsO2=As2O3↓+H2O (3)
Fig.3 shows that the red residue is Cu3(SO3)2·2H2O called the red salt. The reduction reaction is as follows according to the products of As2O3 and Cu3(SO3)2·2H2O:
3Cu3(AsO2)2+3SO2+6H2O=
Cu3(SO3)2·2H2O↓+6HAsO2+H2SO4 (4)
The theoretic content of Cu in Cu3(SO3)2·2H2O is 45.02%. The copper content of the red residue is 30.31% according to Table 3. The copper content of the red residue is lower than the theoretic content because there are some impurities, such as Ca, Zn and Cd, in the residue.
3.3 Preparation of copper sulfate
3.3.1 Influences of reaction time on leaching rates of copper and arsenic
Copper sulfate must be recovered from the red residue in order to decrease the cost. Cu3(SO3)2·2H2O may be oxidized by oxygen in acidic solution to turn into copper sulfate.
The influences of reaction time on leaching rates of Cu and As are shown in Fig.4 when 50 g red residue is added into 24% H2SO4 solution by pumping air under agitating, n(H2SO4)?n(Cu) (molar ratio of H2SO4 to Cu of the residue) is 2.28, and the temperature is 80 ℃.
It can be seen from Fig.4 that the leaching rates increase with the increase of reaction time. The leaching rates of Cu and As are 87.57% and 84.54%, respectively when the reaction time is 0.5 h. The leaching rates of Cu and As reach 99.38% and 91.17% respectively when the time is more than 1.5 h. Therefore, the appropriate reaction time is 1.5 h.

Fig.4 Influences of reaction time on leaching rates of Cu and As
Cu+ and SO32- are oxidized by oxygen to become Cu2+ and SO42- respectively, so Cu3(SO3)2·2H2O in the red residue is dissolved gradually in H2SO4 solution. The reactions are listed as follows:
Cu3(SO3)2·2H2O![]() 2Cu++Cu2++2SO32-+2H2O (5)
2Cu++Cu2++2SO32-+2H2O (5)
4Cu++O2+4H+=4Cu2++2H2O (6)
2SO32-+O2=2SO42- (7)
The total reaction is as follows:
2Cu3(SO3)2·2H2O+2H2SO4+3O2=6CuSO4+6H2O (8)
At the same time, the arsenic in red residue is leached gradually with Cu3(SO3)2·2H2O dissolving. The concentrations of Cu and As are respectively up to 50-60 g/L and 3-4 g/L in the leached solution.
3.3.2 Influences of n(H2SO4)?n(Cu) on leaching rates of copper and arsenic
The influences of n(H2SO4)?n(Cu) on the leaching rate of Cu and As are shown in Fig.5 when the reaction time is 1.5 h and the other conditions above are fixed.
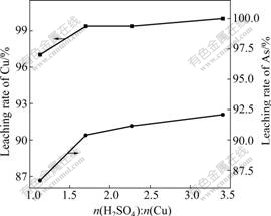
Fig.5 Influences of n(H2SO4)?n(Cu) on leaching rates of Cu and As
It can be seen from Fig.5 that the leaching rates increase with the increase of n(H2SO4)?n(Cu). The leaching rates of Cu and As are 97.01% and 86.67%, respectively when n(H2SO4)?n(Cu) is 1.13. The leaching rates of Cu and As reach to 99.37% and 90.39% respectively when it is more than 1.70. Therefore, the appropriate n(H2SO4)?n(Cu) is 1.70.
The theoretic molar ratio of H2SO4 to Cu is 1?3 according to the reaction above. In fact, the excessive H2SO4 needs to be added in the reaction.
3.3.3 Influences of reaction temperature on leaching rates of copper and arsenic
The influences of reaction temperature on the leaching rate of Cu and As are shown in Fig.6 when n(H2SO4)?n(Cu) is 1.70 and other conditions above are fixed.
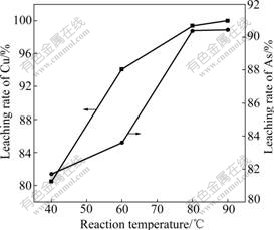
Fig.6 Influences of reaction temperature on leaching rates of Cu and As
It can be seen from Fig.6 that the leaching rates increase with the increase of reaction temperature. The leaching rates of Cu and As reach 99.37% and 90.39% respectively when the reaction temperature is higher than 80 ℃. Therefore, the appropriate reaction temperature is 80 ℃.
3.3.4 Recovery of copper sulfate
The appropriate leaching conditions of the red residue are that the reaction time is 1.5 h, n(H2SO4)?n(Cu) is 1.70, H2SO4 concentration is 24% and the reaction temperature is 80 ℃. 500 g red residue was leached according to the appropriate leaching conditions above to obtain 2.15 L leaching solution in which the concentrations of Cu2+ and As(Ⅲ) were respectively 69.40 g/L and 4.79 g/L. The leaching rates of Cu and As are respectively up to 99.00% and 89.47%.
470 g copper sulfate was prepared from 2.5 L leached solution by evaporating, cooling and crystallizing. The content of CuSO4·5H2O was 98.33%. The direct recovery rate of copper sulfate is up to 79.11%. The total recovery rate may be up to 99.00%, which should equal to the leaching rate. Copper sulfate used to precipitate As in the wastewater can be recovered.
The XRD pattern of the leached residue of H2SO4 is shown in Fig.7. It can be seen from Fig.7 that the leached residue is calcium sulfate, and Cu3(SO3)2·2H2O disappears after leaching.
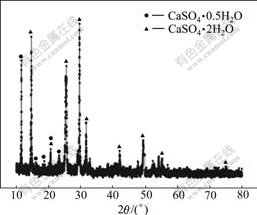
Fig.7 XRD pattern of leached residue
4 Conclusions
(1) The precipitation rate of As is 99.5% and copper arsenite is prepared from As-containing after copper sulfate is added into the As-containing wastewater when the molar ratio of Cu to As is 2?1 and the pH value of the wastewater is adjusted to 8.0 by solid NaOH .
(2) The arsenious acid solution and the red residue are produced after SO2 reduce copper arsenite. The XRD results show that the red residue is Cu3(SO3)2·2H2O and the white powder is As2O3. The reduction reaction is as follows:
3Cu3(AsO2)2+3SO2+6H2O=
Cu3(SO3)2·2H2O↓+6HAsO2+H2SO4
(3) The leaching rate of Cu reaches 99.00% after the red residue is leached by H2SO4 under the action of air when the leaching time is 1.5 h, the molar ratio of H2SO4 to Cu is 1.70, the H2SO4 concentration is 24% and the leaching temperature is 80 ℃. The leaching reaction is as follows:
2Cu3(SO3)2·2H2O+2H2SO4+3O2=6CuSO4+6H2O
(4) The content of CuSO4·5H2O is 98.33%, which is prepared from leached solution by evaporating, cooling and crystallizing. The direct recovery rate of copper sulfate is up to 79.11%.
References
[1] DUTR? V, VANDECASTEELE C. Solidification/stabilisation of hazardous arsenic containing waste from a copper refining process [J]. Journal of Hazardous Materials, 1995, 40(1): 55-68.
[2] LEIST M, CASEY R J, CARIDI D. The management of arsenic wastes: Problems and prospects [J]. Journal of Hazardous Materials, 2000, 76(1): 125-138.
[3] RIOS-ARANA J V, WALSH E J, GARDEA-TORRESDEY J L. Assessment of arsenic and heavy metal concentrations in water and sediments of the Rio Grande at El Paso–Juarez metroplex region [J]. Environment International, 2004, 29(7): 957-971.
[4] HELSEN L, van den BULCK E. Review of disposal technologies for chromated copper arsenate (CCA) treated wood waste with detailed analyses of thermochemical conversion processes [J]. Environmental pollution, 2005, 134(2): 301-314.
[5] RIVEROS G, UTIGARD T A. Disposal of arsenic in copper discharge slags [J]. Journal of Hazardous Materials, 2000, 77(1/3): 241-252.
[6] ALEKSAN D, WITKIEWICZ K, BAL W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis [J]. Toxicology Letters, 2006, 162(1): 29-42.
[7] SHIH C J, LIN C F. Arsenic contaminated site at an abandoned copper smelter plant: Waste characterization and solidification/ stabilization treatment [J]. Chemosphere, 2003, 53(7): 691-703.
[8] SONG S, VALDIVIESO A L, CAMPOS D J H. Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite [J]. Water Research, 2006, 40(2): 364-372.
[9] SHAABAN E R. Optical characterization of arsenic sulfide semiconducting glass films using the transmittance measurements [J]. Materials Chemistry and Physics, 2006, 100(2/3): 411-417.
[10] JAY J A, BLUTE N K, HEMOND H F. Arsenic-sulfides confound anion exchange resin exchange resin speciation of aqueous arsenic [J]. Water Research,2004, 38(5): 1155-1158.
[11] MIHAJLOVIC I, STRBAC N, ZIVKOVIC Z, KOVACEVIC R, STEHERNIK M. A potential method for arsenic removal from copper concentrates [J]. Minerals Engineering,2007, 20(1): 26-33.
[12] LIU Jian-chao, HE Hong-wu, FENG Xin-min. Direction of chemical pesticide development green chemical pesticides [J]. Pesticides, 2005, 44(1): 1-3.
[13] GUAN Yu-jiang, CHEN Liu-chen. Study on the treatment of wastewater from sulphuric acid production by lime PFS process [J]. Environmental Protection of Chemical Industry, 1999, 19(6): 328-335. (in Chinese)
[14] CHAN B K C, DUDENEY A W L. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates [J]. Minerals Engineering,2008, 21(4): 272-278.
[15] NISHIMURA T, ITOH C T, TOZAWA K. Stability and solubilities of metal arsenates and arsenates in water and effect of sulfate and carbonate ions on their solubilities[C]// REDDY R G, HENDRIX J L, QUENEAU P B. Arsenic Fundamentals and Applications. Warrendale, PA: TMS, 1988: 77-97.
[16] ZHENG Ya-jie, WANG Yong, ZHAO Pan-feng. A method of producing arsenite copper and arsenate copper from waste acid contained As: CN, 200610031980.7 [P]. 2006-10-25. (in Chinese)
[17] ZHENG Ya-jie, XIAO Fa-xin, WANG Yong. Preparation and application of copper arsenite: CN, 200610031980.7 [P]. 2006-07- 19. (in Chinese)
[18] WANG Yong, ZHAO Pan-feng, ZHENG Ya-jie. Preparation of copper arsenite from waste acid containing arsenic and its application in copper electrolyte purification [J]. Journal of Central South University: Science and Technology, 2007, 38(6): 1115-1120. (in Chinese)
[19] KHANDAKER N R, TETER D M, KRUMHANSL J L. Arsenic removal in conjunction with lime softening: US 6802980B1 [P]. 2004-12-12.
[20] ZHENG Ya-jie, LUO Yuan, WANG Yong. Method of preparing arsenic trioxide from As-containing wastewater: CN, 200710035704.2 [P]. 2007-09-07. (in Chinese)
Received date: 2008-08-06; Accepted date: 2008-11-17
Corresponding author: ZHENG Ya-jie, Professor, PhD; Tel: +86-731-8836285; E-mail: zzyyjj01@yahoo.com.cn
(Edited by YANG You-ping)
Abstract: The solid sodium hydroxide neutralized acidic As-containing wastewater till pH value was 6. Green copper arsenite was prepared after copper sulfate was added into the neutralized wastewater when the molar ratio of Cu to As was 2?1 and pH value of the neutralized wastewater was adjusted to 8.0 by sodium hydroxide. The arsenious acid solution and red residue were produced after copper arsenite mixed with water according to the ratio of liquid to solid of 4?1 and copper arsenite was reduced by SO2 at 60 ℃ for 1 h. The white powder was gained after the arsenious acid solution was evaporated and cooled. Copper sulfate solution was obtained after the red residue was leached by H2SO4 solution under the action of air. The results show that red residue is Cu3(SO3)2?2H2O and the white powder is As2O3. The leaching rate of Cu reaches 99.00% when the leaching time is 1.5 h, molar ratio of H2SO4 to Cu is 1.70, H2SO4 concentration is 24% and the leaching temperature is 80 ℃. The direct recovery rate of copper sulfate is 79.11% and the content of CuSO4?5H2O is up to 98.33% in the product after evaporating and cooling the copper sulfate solution.


