
Grain refinement of AZ31 magnesium alloy by new Al-Ti-C master alloys
HAN Guang(韩 广), LIU Xiang-fa(刘相法), DING Hai-min(丁海民)
Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials, Ministry of Education,
Shandong University, Ji’nan 250061, China
Received 23 September 2008; accepted 10 March 2009
Abstract:
New Al4C3-containing Al-Ti-C master alloys (Al-0.6Ti-1C and Al-1Ti-1C) were developed by introducing Ti element into Al-C melt using melt reaction method, in which most of the TiC particles distribute around Al4C3 particles. It is believed that most of the C firstly reacts with Al melt and form Al4C3 particles by the reaction Al(l)+C(s)→Al4C3(s), and after adding Ti into the Al-C melt, the size of Al4C3 particles is decreased and the distribution of Al4C3 is improved through the reaction Ti(solute)+Al4C3(s)→TiC(s)+Al(l). With the addition of 1% Al-1Ti-1C master alloy, the average grain size of AZ31 is reduced sharply from 850 μm to 200 μm, and the grain morphology of α-Mg transits from a fully-developed equiaxed dendritic structure to a petal-like shape. Al-C-O-Mn-Fe compounds are proposed to be potent nucleating substrates for primary Mg. Appropriate addition of Ti is believed to increase the grain refinement efficiency of Al4C3-containing Al-Ti-C master alloys in AZ31 alloy.
Key words:
Mg-Al based alloys; grain refinement; Al-Ti-C master alloys; Al4C3; Al-C-O-Mn-Fe compounds;
1 Introduction
Magnesium alloys, as excellent lightweight structural materials, are receiving increasing attention on replacing other metals in automobile and aerospace industries, but their mechanical properties and processing performances can still not meet the requirements of some important parts in the above-mentioned application fields. It is well known that grain refinement is an important technology to improve the mechanical properties, microstructure uniformity and workability of both cast and wrought magnesium alloys[1-3].
Mg-Al based alloys are the most common and commercial magnesium alloys to which many grain refining methods have been applied to obtain a homogeneous and fine microstructure, such as superheating, carbon inoculation, Elfinal process and addition of solute elements[1, 3]. Among these methods, carbon inoculation is known to be the most effective for operating at a low temperature and less fading with long-time holding[1-2]. Amounts of carbon-containing agents such as C2Cl6[4-5], SiC[6-7], Al4C3[8] and carbon powder[7, 9] have been reported to successfully refine Mg-Al based alloys. Among them, C2Cl6 is the most useful grain refiner for commercial application; however, it is prohibited because of causing serious environmental problems for releasing toxic gas during refining. So a reliable, effective and eco-friendly grain refiner should be developed. Among the agents of grain refining, adding master alloys, which can constitute heterogeneous nucleation sites for primary phases and thus cause grain refinement, attracts much attention for important practical advantages such as simple technological operation, effective process control, stable grain refining effect, high environmental safety and application for large melt volumes.
Recently, in the previous work of our research group, PAN et al[10-11] fabricated Al-1C master alloy by melt in-situ reaction, which could efficiently refine AZ63B. However, as Al4C3 has a tendency to aggregate in the melt, Al-C melt always has such a large viscosity that it is very difficult to pour out all of the melt, and also severe stirring is needed to make the particles distribute evenly in the master alloys. What is more, LIU et al[12] found that Al-4Ti-1.5C master alloy fabricated using melt in-situ reaction method could refine Mg-Al based alloys, and it was also noted that regulating the Ti-to-C ratio and improving the proportion of Al4C3 particles could lead to higher grain refining efficiency. Therefore, in this work, Ti element was introduced into Al-C melt to improve the fluidity of the melt and the distribution of Al4C3 in the alloys, and correspondingly a series of Al-Ti-C master alloys with low Ti-to-C ratios were prepared by melt reaction method. Their microstructures and refining performance on AZ31 were studied and the possible refining mechanism was investigated.
2 Experimental
Three types of Al-Ti-C master alloys (namely, Al-0.6Ti-1C, Al-1Ti-1C and Al-1.6Ti-0.4C) were prepared in a graphite crucible using a medium- frequency induction furnace. The chemical compositions of these master alloys are listed in Table 1. A certain amount of carbon-containing preforms are added into the molten Al at 800-1 000 ℃ and held for 10 min. Then different amounts of Al-10Ti master alloy were plunged into the Al-C melt and held for another 10 min. The melt was then stirred sufficiently and poured into a steel mold. Phase identification of the master alloy was performed by X-ray diffraction(XRD) (Rigaku D/max-rB, Japan), and analysis of the microstructures was conducted by electron probe microanalysis(EPMA) (JXA-8800R, Japan).
Table 1 Chemical compositions of Al-Ti-C master alloys (mass fraction, %)
AZ31 alloy was used for grain refinement test and its chemical composition is listed in Table 2. The alloy was melted and heated up to 800 ℃ in stainless steel crucibles coated with MgO using an electric furnace under the protection of flux. 1% (mass fraction) different kinds of Al-Ti-C master alloys were added into the molten AZ31, respectively. After being held for 30 min, the melt was cooled down to 750 ℃ and stirred vigorously, and then cast in a cylindrical steel mold (preheated to 80 ℃) with a size of d 25 mm×60 mm. As Al was brought into the AZ31 melt when adding the Al-Ti-C master alloys, a sample was added 1% commercial pure Al under the same condition for comparison of grain size.
Table 2 Chemical composition of commercial AZ31 magnesium alloy (mass fraction, %)
In order to reveal grain boundaries, the ingots of AZ31 were held at 420 ℃ for 7 h in an air furnace and then water-cooled (T4 treatment). All samples were taken from the center of the ingots. For optical microscopy observation, all specimens both in as-cast state and solid- solution state were sectioned, mounted, polished and etched in a solution of picric and acetic acid. Microstructures were observed using a high scope video microscope (HSVM) (KH-2200MD2, Japan) and EPMA, and the grain sizes of the ingots were measured by applying the linear-intercept method.
3 Results and discussion
3.1 Microstructures of Al-Ti-C master alloys
Fig.1 shows the XRD patterns of the fabricated Al-Ti-C master alloys. Evident peaks of Al4C3, TiC and Al are detected in the XRD patterns of Al-0.6Ti-1C and Al-1Ti-1C master alloys, while only peaks of TiC and Al are found in that of Al-1.6Ti-0.4C master alloy.
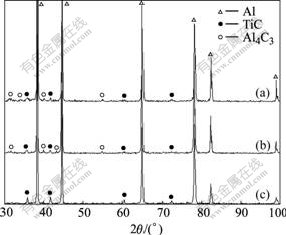
Fig.1 XRD patterns of Al-0.6Ti-1C (a), Al-1Ti-1C (b) and Al-1.6Ti-0.4C (c) master alloys
The microstructures of these Al-Ti-C master alloys are presented in Fig.2. A kind of black phase is distributed in Al matrix in Al-0.6Ti-1C and Al-1Ti-1C master alloys, surrounded by a kind of small white particles. It is observed that the black phase has a tendency to aggregate especially in Al-0.6Ti-1C, and the aggregation of this phase becomes quite slighter in Al-1Ti-1C, of which the number of white particles increases. Besides, there are some white particles distributed separately in the Al matrix of these two Al-Ti-C. Different from these two master alloys, the phase located in Al matrix in Al-1.6Ti-0.4C is almost the white phase, and few black phase (marked by the arrow) also surrounded by white particles is observed. Therefore, combining the EPMA micrographs with the XRD patterns of the Al-Ti-C master alloys, it can be primarily deduced that the black phase is Al4C3, and the white is TiC.
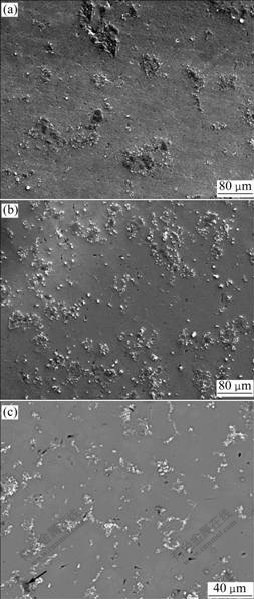
Fig.2 EPMA micrographs of Al-0.6Ti-1C (a), Al-1Ti-1C (b) and Al-1.6Ti-0.4C (c) master alloys
EPMA mapping analysis of the particles in Al-1Ti-1C master alloy is shown in Fig.3. It is shown more clearly in Fig.3(a) that some white particles with diameters of 0.5-1.5 μm are located around a kind of black polygonal particles with sizes of 4-10 μm. It can be seen that the white particles contain Ti and C, indicating they are TiC particles, while the black phase enriches in Al and C, indicating they are Al4C3 particles.
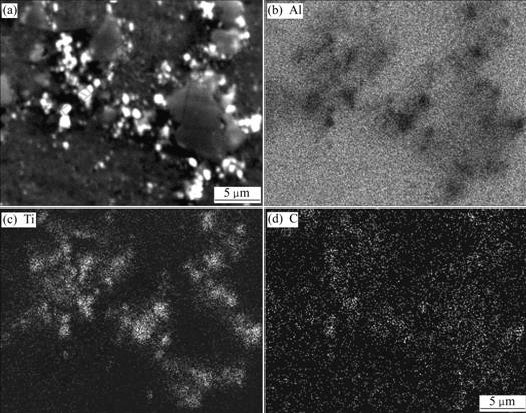
Fig.3 EPMA mapping analysis of Al-1Ti-1C master alloy: (a) SEI image; (b)-(d) Distribution of Al, Ti and C
Fig.4 shows the EPMA micrographs of TiC and Al4C3 particles in Al-0.6Ti-1C and Al-1Ti-1C master alloys under higher magnification. Al4C3 particles have a size of 15-30 μm and 4-10 μm in the Al-0.6Ti-1C and Al-1Ti-1C, respectively. Al4C3 particles aggregate severely and always form particle clusters with a relatively large size in Al-0.6Ti-1C. The aggregation of Al4C3 particles becomes slighter evidently and Al4C3 phase is separated by TiC particles into particles with a much smaller size in Al-1Ti-1C compared with Al-0.6Ti-1C. So, it can be concluded that the addition of Ti element influences the size and distribution of Al4C3 particles.

Fig.4 EPMA micrographs of TiC and Al4C3 particles in Al-0.6Ti-1C (a) and Al-1Ti-1C (b) master alloys
3.2 Forming mechanism of Al4C3 and TiC in Al-Ti-C master alloys
The melt reactions during the fabrication of Al-Ti-C master alloys containing TiC and TiAl3 by melt in-situ reaction method have been studied by many researchers [13-16]. It is supposed that the possible reactions that influence the formation of Al4C3 and TiC phases are
Al(l)+C(s)→Al4C3(s) (1)
Ti(solute)+C(s)→TiC(s) (2)
Ti(solute)+Al4C3(s)→TiC(s)+Al(l) (3)
According to Ref.[13] and Ref.[15], reaction (1) is available at about 705 ℃ and the reaction (3) can occur at about 890 ℃. Moreover, a temperature above 1 100 ℃ is needed for the initiation of reaction (2), so that the Ti(solute) and C(s) can react efficiently.
In this work, the fabrication procedures of Al-Ti-C master alloys consist of two key steps, that is, the fabrication of Al-C melt and the adding of Ti element into the Al-C melt. For the synthesis temperature is at 800-1 000 ℃, the reactions (1) and (3) can occur efficiently, but it is impossible for the acute processing of reaction (2). At the stage of fabricating Al-C melt, it is assumed that most of the C first reacts with Al melt to form Al4C3 after plunging the C-containing preforms into Al melt. It is observed that the melt has a sharp increase in viscosity, indicating the abundant formation of Al4C3. After adding Al-10Ti master alloy into the Al-C melt, the initiation condition of reaction (3) is fulfilled, and Ti solute will diffuse to the interface of Al4C3 phase and react with Al4C3 particles to form TiC particles. From the EPMA micrographs, it is observed that Al4C3 particles are surrounded by TiC particles in the Al-0.6Ti-1C and Al-1Ti-1C master alloys, and even in Al-1.6Ti-0.4C master alloy the few remaining Al4C3 are surrounded by TiC, indicating that the TiC is formed on the interface of Al4C3 particles through reaction (3), which is a direct evidence for the assumption of forming mode of Al4C3 and TiC in our experiments.
As a result of the formation of TiC on the surface of Al4C3 phase, Al4C3 particles are “lick up” by the solute Ti, and many Al4C3 particles are separated into smaller ones as shown in Fig.4(b). Therefore, it is deduced that the formation of TiC particles in this way decreases the sizes of Al4C3 particles and improves the distribution of Al4C3. As Ti content increases in the Al-1Ti-1C master alloy, more sufficient formation of TiC takes part in the size decrease of Al4C3 particles. As a result, in contrast to Al-0.6Ti-1C, the sizes of Al4C3 particles in Al-1Ti-1C sharply decease from 15-30 μm to less than 10 μm and the aggregation of Al4C3 particles becomes slighter evidently. Moreover, although there are only very small amount of Al4C3 particles in the Al-1.6Ti-0.4C, these particles have also a relatively small size and are surrounded by TiC particles, indicating the formation of TiC functions in the size decreasing of Al4C3. So, the formation of TiC particles plays an important role in improving the final sizes and distribution of Al4C3 particles in Al4C3-containing Al-Ti-C master alloys.
Another thing to mention is that there are few separate TiC particles distributed in the Al matrix. So, it is believed that reaction (2) also occurs, that is, some residue carbon can react with Ti solute; however, the reaction is rather weak.
3.3 Grain refinement efficiency of Al-Ti-C master alloys in AZ31 alloy
The microstructures of as-cast AZ31 with and without the addition of grain refiners are presented in Fig.5. Fig.5(a) shows that AZ31 has a typical equiaxed dendritic structure with inter-dendritic angle of 60? and many nonequilibrium eutectic phases formed in the inter-dendritic region. In contrast to the sample added with 1% commercial pure Al (Fig.5(b)), adding 1% Al-1.6Ti-0.4C (Fig.5(e)) does not show any evident grain refinement, and the first dentrites are still well developed. As Figs.5(c) and (d) show, the decrease of grain size as well as the transition of primary phases from a fully developed sixford symmetrical dendrite structure to a less developed dendrite structure after adding 1% Al-0.6Ti-1C or Al-1Ti-1C is readily noticeable. Between the two Al4C3-containing Al-Ti-C master alloys mentioned above, Al-1Ti-1C shows the better grain refining efficiency. When adding 1% Al-1Ti-1C (Fig.5(d)), the grain size of AZ31 is reduced to nearly 1/3 that of the sample added with 1% commercial pure aluminum, and the grain morphology of α-Mg transits from a characteristic sixfold symmetrical shape to a petal-like shape; when adding 1% Al-0.6Ti-1C (Fig.5(c)), the grain size of AZ31 is reduced to nearly 1/2 that of the sample added with 1% commercial pure aluminum, and the first dentrites become undeveloped.

Fig.5 Optical micrographs of as-cast AZ31 alloy with addition of no master alloy (a), 1% commercial pure Al (b), 1% Al-0.6Ti-1C (c), 1% Al-1Ti-1C (d) and 1% Al-1.6Ti-0.4C (e)
Fig.6 presents the microstructures of AZ31 in T4 solid solution state with and without the addition of grain refiners, and Fig.7 shows the corresponding grain sizes of AZ31 calculated by the linear-intercept method. The average grain size of AZ31 without the grain refiner is nearly 850 μm; the average grain sizes by adding 1% commercial pure Al and Al-1.6Ti-0.4C master alloys are 550 μm and 500 μm, respectively. When adding 1% Al-1Ti-1C and Al-0.6Ti-1C master alloys, the average grain size is decreased to 200 μm and 340 μm, respectively. So the Al-1Ti-1C master alloy shows the most efficient grain refinement on AZ31.
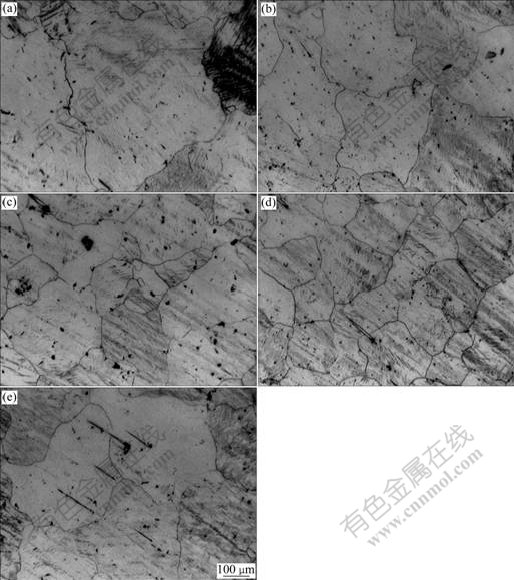
Fig.6 Optical micrographs of AZ31 in T4 solid solution state with addition of no master alloy (a), 1% commercial pure Al (b), 1% Al-0.6Ti-1C (c), 1% Al-1Ti-1C (d) and 1% Al-1.6Ti-0.4C (e)
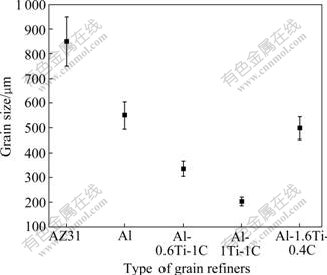
Fig.7 Grain sizes of AZ31 in T4 solid solution state with and without addition of grain refiners
3.4 Grain refining mechanism of Al-Ti-C master alloys in AZ31 alloy
Grain refinement usually involves adding both special alloying elements and potent foreign nucleants into the melt. The effect of solute elements on grain refinement is to generate constitutional undercooling in the diffusion layer ahead of the advancing S/L interface and restrict the grain growth by slowing down the diffusion of the solute, which is explained in terms of the growth restriction factor(GRF). Alloying element Al has been found to refine the pure magnesium obviously because of its relatively larger GRF value, and the grain size of Mg-Al alloys decreases sharply with the increase of Al content when the content of Al is lower than 5%[1]. In this work, adding 1% commercial pure Al (AZ41) causes mild grain refinement on AZ31; however, adding 1% Al-1Ti-1C which contains nearly the same amount of Al can refine the grains further to a larger degree. Hence, the extra Al brought by adding the master alloy is not likely to play a dominant role in the grain refinement.
What is more, adding potent foreign nucleants into the melt will decrease the nucleation energy and increase the nucleation frequency, which can also lead to grain refinement. It can be seen that Al-1Ti-1C shows excellent grain refining efficiency on AZ31 after eliminating the influence of constitutional undercooling generated by extra Al. It is hence reasonable to suggest that Al4C3 or TiC particles brought by adding the Al-1Ti-1C are important for the nucleation of a-Mg. Moreover, grain refinement experiments reveal that Al-1.6Ti-0.4C containing almost only TiC particles does not show any evident grain refinement on AZ31, indicating TiC particles are not good grain refiners for AZ31 in this work, and Al4C3 particles may play a key role in the grain refinement.
It is believed that as soon as α-Mg nucleates on a potent nucleating substrate in the melt, it will grow radially in a uniform fashion[8]. As a result, an effective nucleant particle is commonly expected in the central regions of α-Mg grains. In addition to the decrease of grain size and the morphology transition of α-Mg, it is also observed that there are some tiny particles (marked by the arrow) situated in the center of a-Mg in Fig.5(d), which may be the nucleation sites. Figs.8(a) and (b) present typical back-scattered electron(BSE) images of AZ31 alloy in as-cast state refined by 1% Al-1Ti-1C master alloy, which clearly reveals a tiny particle in the center of α-Mg that may act as the nucleation site. EPMA line analysis across the particle shows that it enriches in Al, C, O, Mn, Fe and no Ti. This indicates that TiC is not a grain refiner and does not contribute directly to the nucleation, and Al4C3, Mn and Fe play an important role in the nucleation of α-Mg.
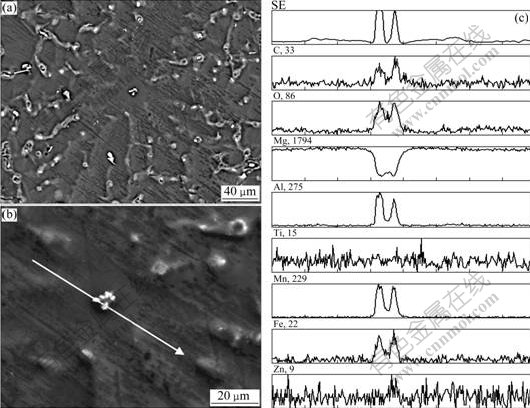
Fig.8 EPMA line analysis of AZ31 refined by 1% Al-1Ti-1C master alloy: (a) BSE image of a complete α-Mg grain; (b) BSE image of particle in center of α-Mg; (c) Line analysis across particle in Fig.8(b)
In the previous work of our research group, PAN et al[10,17] proved that Al-C-O-Fe-Mn-rich particles could nucleate α-Mg and a two-phase nucleation theory was proposed that the combination of Al-C-O-rich and Al-Mn-Fe-rich compounds produced more powerful nuclei of α-Mg. Being consistent with the previous results, the present study shows that Al-C-O-Mn-Fe particles can act as the nucleation sites of α-Mg, and it is believed that Al4C3 particles react and combine with Al-Mn-Fe particles to form powerful nuclei of α-Mg. What is more, it should be noted that Al-0.6Ti-1C containing Al4C3 particles with a size of 15-30 μm which is relatively large show less efficient grain refinement compared with Al-1Ti-1C, indicating either less Al4C3 particles combine with Mn and Fe to form efficient Al-C-O-Mn-Fe nucleate particles or the formed Al-C-O-Mn-Fe particles have lower nucleating potency than that formed by adding Al-1Ti-1C. Hence, it is deduced that the size of Al4C3 particles in the Al4C3-containing Al-Ti-C master alloys is a key point for their grain refinement on Mg-Al based alloys, and the addition of Ti plays an important role in the improvement of grain refinement efficiency of the Al-Ti-C master alloys.
4 Conclusions
1) New Al4C3-containing Al-Ti-C master alloys (Al-0.6Ti-1C and Al-1Ti-1C) were fabricated through introducing Ti element into Al-C melt by melt reaction method, in which Al4C3 particles are surrounded by TiC particles. It is deduced that most of C firstly reacts with Al melt and forms Al4C3 particles through the reaction Al(l)+C(s)→Al4C3(s), and then the reaction Ti(solute)+Al4C3(s)→TiC(s)+Al(l) occurs, leading to the decreased sizes and improved distribution of Al4C3 particles.
2) The Al-1Ti-1C master alloy with 4-10 μm Al4C3 particles can refine AZ31 efficiently, while the Al-0.6Ti-1C with 15-30 μm Al4C3 particles shows less efficient grain refinement. Detailed EPMA observation suggests Al-C-O-Mn-Fe compounds are the nucleating substrates for α-Mg. Appropriate addition of Ti is believed to increase the grain refinement efficiency of Al4C3-containing Al-Ti-C master alloys.
References
[1] LEE Y C, DAHLE A K, StJOHN D H. The role of solute in grain refinement of magnesium [J]. Metallurgical and Materials Transaction A, 2000, 31(11): 2895-2906.
[2] EMLEY E F. Principles of magnesium technology [M]. Oxford: Pergamon Press, 1966: 200-211.
[3] StJOHN D H, QIAN M, EASTON M A, CAO P, HILDEBRAND Z. Grain refinement of magnesium alloys [J]. Metallurgical and Materials Transaction A, 2005, 36(7): 1669-1679.
[4] JIN Q L, EOM J P, LIM S G, PARK W W, YOU B S. Grain refining mechanism of a carbon addition method in a Mg-Al magnesium alloy [J]. Scripta Materialia, 2003, 49(11): 1129-1132.
[5] KIM Y M, YIM C D, YOU B S. Grain refining mechanism in Mg-Al base alloys with carbon addition [J]. Scripta Materialia, 2007, 57(8): 691-694.
[6] G?NTHER R, HARTIG C, BORMANN R. Grain refinement of AZ31 by (SiC)P: Theoretical calculation and experiment [J]. Acta Materialia, 2006, 54(20): 5591-5597.
[7] LU L, DAHLE A K, StJOHN D H. Heterogeneous nucleation of Mg-Al alloys [J]. Scripta Materialia, 2006, 54(12): 2197-2201.
[8] LU L, DAHLE A K, StJOHN D H. Grain refinement efficiency and mechanism of aluminium carbide in Mg-Al alloys [J]. Scripta Materialia, 2005, 53(5): 517-522.
[9] MOTEGI T. Grain-refining mechanisms of superheat-treatment of and carbon addition to Mg-Al-Zn alloys [J]. Materials Science and Engineering A, 2005, 413/414: 408-411.
[10] PAN Yi-chuan, LIU Xiang-fa, YANG Hua. Role of C and Fe in grain refinement of an AZ63B magnesium alloy by Al-C master alloy [J]. Journal of Materials Science and Technology, 2005, 21(6): 822-826.
[11] PAN Yi-chuan, LIU Xiang-fa, YANG Hua. Grain refinement of an AZ63B magnesium alloy by an Al-1C master alloy [J]. International Journal of Materials Research, 2005, 96(12): 1398-1403.
[12] LIU Yan-hui, LIU Xiang-fa, LI Ting-bin, BIAN Xiu-fang. Grain refining effect of Al-Ti-C master alloy on Mg-Al alloys [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(3): 622-625. (in Chinese)
[13] KENNEDY A R, WESTON D P, JONES M I, ENEL C. Reaction in Al-Ti-C powders and its relation to the formation and stability of TiC in Al at high temperatures [J]. Scripta Materialia, 2000, 42(12): 1187-1192.
[14] LEE J H, THADHANI N N, GREBE H A. Reaction sintering of shock-compressed Ti+C powder mixtures [J]. Metallurgical and Materials Transactions A, 1996, 27(7): 1749-1759.
[15] WANG Zhen-qing, LIU Xiang-fa, ZHANG Jun-yan, BIAN Xiu-fang. Reaction mechanism in the ball-milled Al-Ti-C powders [J]. Journal of Materials Science Letters, 2003, 22(20): 1427-1429.
[16] RAPP R A, ZHENG X J. Thermodynamic consideration of grain refinement of aluminum alloy by titanium and carbon [J]. Metallurgical and Materials Transactions A, 1991, 22 (12): 3071-3075.
[17] PAN Yi-chuan. Heterogeneous nucleation of α-Mg, Mg2Si in Mg alloys and development of relevant master alloys [D]. Jinan: Shandong University, 2006: 94-98. (in Chinese)
Foundation item: Project(50625101) supported by the National Natural Science Foundation for Distinguished Young Scholars of China; Project(106103) supported by Key Project of Science and Technology Research of Ministry of Education of China
Corresponding author: LIU Xiang-fa; Tel: +86-531-88392006; E-mail: xfliu@sdu.edu.cn
DOI: 10.1016/S1003-6326(08)60406-9


