
Controlling limiting length of tunnels on Al foil electroetched in
HCl-H2SO4 solution
BAN Chao-lei(班朝磊), HE Ye-dong(何业东)
Beijing Key Laboratory for Corrosion, Erosion and Surface Technology,
University of Science and Technology Beijing, Beijing 100083, China
Received 23 September 2008; accepted 17 January 2009
Abstract:
The limiting length of tunnels, llim, of Al foil electro-etched in HCl-H2SO4 solution and the corresponding anodic polarization curves in the same solution were measured. It is found that there is a dependence of llim on the potential difference, ?φ, between the pitting potential, φpit, and the corrosion potential, φcorr, of Al foil, when the temperature and H2SO4 concentration of HCl-H2SO4 electrolyte are changed. The dynamic equation on the tunnel growing and the linear equation between llim and ?φ were deduced by analyzing the relationship among the over-potential on Al foil surface, the transport over-potential in tunnel solution and the over-potential at tunnel tip during the electro-etching. The results show that the growing velocity of tunnels decreases with their extending in length and the changing trend of llim can be judged by measuring ?φ, which supplies a convenient access to explore new kinds of etchants.
Key words:
electrolytic capacitor; aluminum foil; electro-etching; anodic polarization; pitting;
1 Introduction
Electrochemical etching of aluminum foil can enlarge its surface area and is a key technology to manufacture Al electrolytic capacitor of high special capacitance. The etched foil for high voltage Al electrolytic capacitor is fabricated by d.c. electrochemical etching in concentrated chloride solutions to produce crystallographic tunnels along <100> direction on high purity and high cubicity Al foil surface[1]. Their surface density is of the order of 105-107 /cm2. The tunnel etching of Al foil is a typical progress of pitting corrosion. It not only depends on properties of raw foil[2-5], but also involves the initiation and growth of pits and tunnels, mass transportation in tunnels, dissolution and passivation of Al, chemical equilibrium and self-catalysis in tunnels, etc [6-8]. In 1984, ALWITT et al[9] first reported the dynamics of tunnel growth for Al foil in 1 mol/L HCl at 70-97 ℃. They found that tunnel initially increased at a constant rate and developed slowly later in length, eventually reached a limiting length where they no longer grew under given etching conditions. Subsequently, the conception of tunnel limiting length, llim, was defined by HEBERT and ALKIRE[10]. Their research showed that llim decreased with increasing concentrations of AlCl3, HCl or temperature when Al foil was d.c. electro-etched in HCl solution. They thought that the resistance to transport of Al+3 from tunnel tip to outer bulk solution increased with increasing tunnel length and diffusion became the controlling step for tunnel growing. When tunnel became saturated with AlCl3 at its tip, the tunnel tip would passivate and stop growing, leading to a limiting tunnel length. ATSUSHI[11] studied the influence of H2SO4 concentration in 1 mol/L HCl at 90 ℃ on llim. He found that llim trended to decrease with increasing H2SO4 concentration. It was suggested that Al2(SO4)3 could reach a saturated state earlier than AlCl3 at tunnel tips because the solubility of Al2(SO4)3 was less than that of AlCl3, resulting in the inhibitation of the growth of tunnels. In 1996, a local chemical process during tunnel etching Al foil was analyzed by NEWMAN[12]. In China, WANG et al[13] and WANG et al[14] have also studied the factors influencing llim. They proposed that the passivation of H2SO4 and mass transport process in the boundary layer on the Al foil surface had an important effect on llim.
In this work, the dependence of llim on the potential difference, ?φ, between the pitting potential and the corrosion potential of Al foil during electro-etching in HCl+H2SO4 solution was studied. Through experiments and theoretical analysis, the relation of this dependence with the mechanism of controlling llim was revealed.
2 Experimental
Aluminum capacitor grade foil (Showa) of 99.99% in purity, 115 μm in thickness and above 99% in cubicity for high voltage usage was adopted as specimen.
Al foil specimens were rinsed with deionized water and mounted in a holder, leaving a single exposed surface area of 5 cm×9 cm. A potentio/galvanostat(WYK-3050) supplied the d.c. current for anodic etching. The current density and surface density of charge were 150 mA/cm2 and 20 C/cm2, respectively. Anodic etching was carried out in 1 mol/L HCl+x mol/L H2SO4 solutions (x=0.5, 1, 1.5, 2 and 2.5) in a temperature range from 70 ℃ to 90 ℃.
After anodic etching, Al foils were rinsed with deionized water, and then anodized in a ammonium adipate bath at 90 ℃ under a voltage of 100 V. A anodic film was thus formed. The foils were then immersed in an iodine/methanol solution at 40 ℃ for 10 h, in which the Al matrix was dissolved away, leaving the oxide replica of tunnels for SEM observation. After rinsing and drying, the specimens were fixed and coated with thin carbon film. The morphology of tunnels and llim were observed and measured directly by FE-SEM (SUPRA55).
The anodic polarization curves of Al foil in above etching solutions were measured by a Potentiosat-168. The test cell was a three-electrode system consisting of the anodized aluminum, a platinum sheet and a saturated calomel electrode(SCE) assembled as working electrode, counter electrode and reference electrode, respectively. The reference electrode employed a salt bridge with a Luggin probe adjacent to the working electrode. The period of adopting data was 500 ms. Specimens were anodizedly polarized at a sweep rate of 10 mV/s.
3 Results and discussion
3.1 Effect of H2SO4 concentration on llim and anodic polarization curves
Fig.1 shows the morphologies of oxide replica of tunnels etched in 1 mol/L HCl+x mol/L H2SO4 with different H2SO4 concentrations at 85 ℃. It is demonstrated that llim decreases with increasing H2SO4 concentration. Fig.2 shows the anodic polarization curves of Al foil specimens in the corresponding solutions and ?φ. By comparing Fig.1 with Fig.2, it can be seen that both llim and ?φ decrease with increasing H2SO4 concentration.
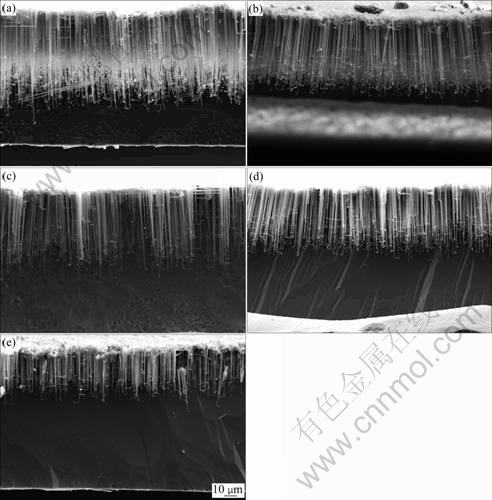
Fig.1 Morphologies of oxide replica of tunnels etched in 1 mol/L HCl+x mol/L H2SO4 solution: (a) x=0.5; (b) x=1.0; (c) x= 1.5; (d) x=2.0; (e) x=2.5

Fig.2 Anodic polarization curves of Al foil in 1 mol/L HCl+ x mol/L H2SO4 solution
3.2 Effect of temperature on llim and anodic polariza- tion curves
Fig.3 and Fig.4 show the tunnel morphologies and anodic polarization curves obtained in 1 mol/L HCl+1.5 mol/L H2SO4 at different temperatures, respectively. It can be seen that both llim and ?φ decrease with increasing solution temperature.
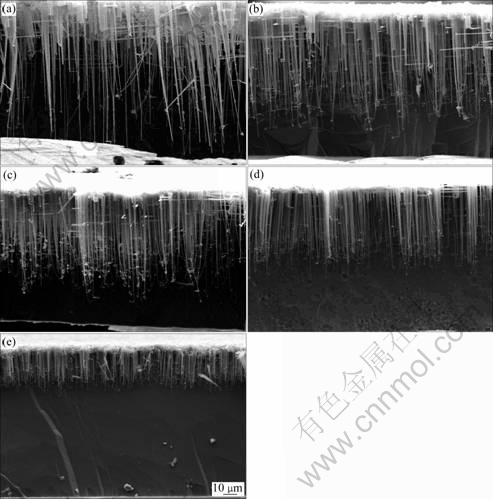
Fig.3 Morphologies of tunnels formed in 1 mol/L HCl+1.5 mol/L H2SO4 solution at different temperatures: (a) 70 ℃; (b) 75 ℃; (c) 80 ℃; (d) 85 ℃; (e) 90 ℃

Fig.4 Anodic polarization curves of Al foil in 1 mol/L HCl+1.5 mol/L H2SO4 solution at different temperatures
From above experimental results, two relation curves of llim with ?φ can be drawn, as shown in Fig.5, when changing H2SO4 concentration or temperature of 1 mol/L HCl+x mol/L H2SO4 solution. Fig.5 shows that llim is approximately linear with ?φ. The nobler the ?φ, the larger the llim. However, the influence of temperature or H2SO4 concentration on the slopes of llim—?φ curves is different. The slope of curve for the effect of temperature is steeper than that of curve for the effect of H2SO4 concentration.
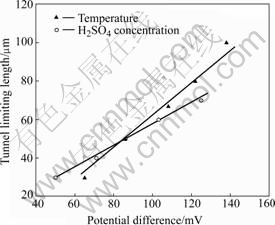
Fig.5 Influences of temperature and H2SO4 concentration of 1 mol/L HCl+x mol/L H2SO4 on relation between potential difference (?φ) and tunnel limiting length (llim)
The tunnel etching of Al foil is a unique form of pitting corrosion. Generally, the value of ?φ reflects the trend for pitting corrosion to occur. With the increase of H2SO4 concentration or temperature of solution, ?φ decreases, indicating that the pitting corrosion of Al foil occurs more easily. Moreover, as shown in Fig.5, there is a dependence of llim on ?φ when H2SO4 concentration or temperature of solution changes. If this dependence is inherent in etching process, the changing trend of llim can be judged through measuring ?φ, which provides a convenient way to explore new kinds of etchants for controlling llim. Therefore, it is very significant to study the inherent mechanism of llim—?φ dependence.
During tunnel growing, the electrochemical behavior in tunnel is different at the exterior surface of Al foil. Both the tunnel walls and the Al foil exterior surface between tunnels are under passivation, and only the tunnel tip could be dissolved. The anodic polarization curves show the potential variation at the mouth of tunnel and Al foil exterior surface with the apparent current density. The potential at the tunnel mouth is always equal to that at Al foil exterior surface. However, the true current densities at both locations are different. As a tunnel grows, the true current density at the tunnel mouth is always larger than that at the Al foil exterior surface until the tunnel stops growing. Since both the tunnel mouth and the Al foil exterior surface are under an isopotential state and the electrochemical behaviors at both locations have a parallel connection, the following relation exists:
φs=φtip+φsol (1)
where φs and φtip are the polarization over-potentials at the Al foil exterior surface and at the tunnel tip, respectively; and φsol is the mass transport over-potential in the tunnel solution.
There are
φsol=Jpit?spit?Rsol (2)
![]() (3)
(3)
where Jpit is the current density in the tunnel; spit is the cross section area of the tunnel; Rsol is the solution resistance in the tunnel; κ is the solution conductivity in the tunnel and lpit is the tunnel length. Eq.(1) can be expressed as
![]() (4)
(4)
From Eq.(4), Jpit can be drawn as
![]() (5)
(5)
When the current density at tunnel tip drops to a critical value, Jc, the tunnel tip becomes passivated and the tunnel reaches llim. The following equation can be obtained:
![]() (6)
(6)
Eqs.(5) and (6) indicate that the driving force for tunnel growth is (φs-φtip). The more the passivity of the Al foil exterior surface, the more the value of (φs-φtip). The dynamics of the tunnel growing can be changed through the passivation ability of Al foil surface. Eqs.(5) and (6) also suggest that Jpit is inversely proportional to the tunnel length if (φs-φtip) keeps constant. This is in agreement with the fact that the Jpit tends to decrease with the increase of the tunnel length, as reported in the Refs.[9, 12, 15]. Eqs.(5) and (6) describe the growth dynamics of one tunnel. During electro-etching, many tunnels are formed closely following others. The applied current density can only change the number of tunnels formed, rather than the dynamics and limiting length of each tunnel.
Owing to the occlusion cell effect in tunnel, the solution at tunnel tips is characterized by special concentrations of H+, Al3+ and Clˉ, which are higher than those in the bulk solution. When the metal dissolution rate of tunnel tip is not high, φtip can be neglected, compared with φsol. Therefore, Eq.(6) can be reduced as
![]() (7)
(7)
Since φs consists of ?φ and ?φ1, the potential difference between the foil exterior surface potential and the pitting potential, namely φs=?φ+?φ1, Eq.(7) can be further expressed as
![]() (8)
(8)
It can be found from Fig.2 and Fig.4 that the influences of both H2SO4 concentration and solution temperature on ?φ1 are small. In addition, because the composition of solution in tunnel is little influenced by bulk solution due to the occlusion cell effect, κ can be regarded as a constant at certain temperature. Therefore, as shown in Eq.(8), llim is linear with ?φ at certain temperature, which is confirmed by the experiment results in Fig.5. When the solution temperature increases, ?φ decreases as shown in Fig.4 with κ increasing in theory. So, as the solution temperature increases, the relationship between llim and ?φ will be deflected from that at constant temperature. As demonstrated in Fig.5, the slope of llim—?φ curve influenced by solution temperature is steeper than that influenced by H2SO4 concentration. Therefore, enhancing solution temperature can apparently decrease the tunnel limiting length.
4 Conclusions
1) When Al foil for high voltage is electro-etched in HCl-H2SO4 solution, there is a linear relationship between llim and ?φ with changing of H2SO4 concentration and temperature of the solution.
2) The dynamic equation on tunnel growing can be drawn as Jpit=κ(φs-φtip)/lpit, which demonstrates that Jpit is in inverse proportion to tunnel length, lpit.
3) When the tunnel tip is under critical passivation current density, Jc, the tunnel will reach a limiting length, llim, which can be expressed as llim=κ(φs-φtip)/Jc, leading to a approximate linear relationship between llim and ?φ for a given experimental conditions, which is confirmed by the experiment results.
4) The changing trend of llim can be judged through measuring ?φ, which supplies a convenient way to explore new kinds of etchants for controlling the tunnel limiting length.
References
[1] ONO S, MAKINO T, ALWITT R S. Crystallographic pit growth on aluminum(100) [J]. Journal of the Electrochemical Society, 2005, 152(2): B39-B44.
[2] MAO Wei-min, YANG Hong, YU Yong-ning, FENG Hui-ping, XU Jin. Influence of trace Mg on corrosion structure of high voltage aluminum foil [J]. The Chinese Journal of Nonferrous Metals, 2003, 13(5): 1057-1059. (in Chinese)
[3] ZHANG Xin-ming, MENG Ya, ZHOU Zhuo-ping, ZHOU Hong-chang. Effects of Fe impurity on recrystallization textures and specific capacitances of high pure aluminium foils [J]. The Chinese Journal of Nonferrous Metals, 1999, 9(1): 19-24. (in Chinese)
[4] MAO Wei-min, CHEN Lei, SA Li-man, YU Yong-ning, LI Yun-feng. Influence of grain boundaries on corrosion structure of low voltage aluminum foil [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(1): 1-5. (in Chinese)
[5] MAO Wei-min, JIANG Heng, YANG Ping, FENG Hui-ping, YU Yong-ning. Influence of microstructure and microelements on corrosion structure of aluminum foil [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1627-1631. (in Chinese)
[6] KEUONG Y W, NORDIEN J H, ONO S, NISANCIOGLU K. Electrochemical activation of aluminum of trace element lead [J]. Journal of the Electrochemical Society, 2003, 150(1): B547-B551.
[7] MUTHUKRISHNAN K, HEBERT K R. Kinetic model for aluminum dissolution in corrosion pits [J]. Journal of the Electrochemical Society, 2004, 151(2): B45-B52.
[8] WALL F D, MARTINEZ M A, VANDENAVYLE J J. Relationship between induction time for pitting and pitting potential for high-purity aluminum [J]. Journal of the Electrochemical Society, 2004,151(6): B354-B358.
[9] ALWITT R S, UCHI H, BECK T R, ALKIRE R C. Electrochemical tunnel etching of aluminum [J]. Journal of the Electrochemical Society, 1984, 131(1): 13-18.
[10] HEBERT K, ALKIRE R C. Growth and passivation of aluminum etch tunnels [J]. Journal of the Electrochemical Society, 1988, 135(11): 2146-2157.
[11] ATSUSHI H. The effect of sulfuric acid on tunnel etching of aluminum in hydrochloric acid [J]. Light Metals, 1992, 42(8): 440-445.
[12] NEWMAN R C. Local chemistry considerations in the tunnelling corrosion of aluminum [J]. Corrosion Science, 1995, 37(3): 527-533.
[13] WANG Gang, YAN Kang-ping, YAN Ji-xin. The influence of H2SO4/HCl ratio and Al3+ content on the tunnel growth of high purity aluminum foil [J]. Electronic Components & Materials, 2003, 22(10): 10-12. (in Chinese)
[14] WANG Mei, HE Ye-dong, HONG Tao. Effects of etching conditions on the limiting tunnel length of anodic Al foil for high voltage [J]. Electronic Components & Materials, 2007, 26(7): 17-20. (in Chinese)
[15] HEBERT K, ALKIRE R. Growth rates of aluminum etch tunnels [J]. Journal of the Electrochemical Society, 1988, 135(12): 2447-2451.
Foundation item: Project supported by University New Materials Disciplines Construction Program of Beijing, China
Corresponding author: HE Ye-dong; Tel: +86-10-62332715; E-mail: htgroup@mater.ustb.edu.cn
DOI: 10.1016/S1003-6326(08)60319-2
Abstract: The limiting length of tunnels, llim, of Al foil electro-etched in HCl-H2SO4 solution and the corresponding anodic polarization curves in the same solution were measured. It is found that there is a dependence of llim on the potential difference, ?φ, between the pitting potential, φpit, and the corrosion potential, φcorr, of Al foil, when the temperature and H2SO4 concentration of HCl-H2SO4 electrolyte are changed. The dynamic equation on the tunnel growing and the linear equation between llim and ?φ were deduced by analyzing the relationship among the over-potential on Al foil surface, the transport over-potential in tunnel solution and the over-potential at tunnel tip during the electro-etching. The results show that the growing velocity of tunnels decreases with their extending in length and the changing trend of llim can be judged by measuring ?φ, which supplies a convenient access to explore new kinds of etchants.


