文章编号:1004-0609(2010)S1-s0953-05
生物医用Ti-Nb基合金的显微组织与耐磨性
王本力1,李 莉1,郑玉峰2
(1. 哈尔滨工程大学 生物医学材料与工程研究中心,哈尔滨 150001;
2. 北京大学 工学院,北京 100871)
摘 要:
采用金相显微镜和X射线衍射仪分析生物医用Ti-22Nb,Ti-25Nb及Ti-22Nb-2Zr/Hf合金的显微组织和相组成,采用圆盘式摩擦实验机和维氏硬度计研究合金的耐磨性,利用扫描电子显微镜观察摩擦后合金的表面形貌,主要考察合金元素(Zr,Hf)和热处理工艺对Ti-Nb基合金耐磨性的影响,分析其摩擦机制。结果表明:钛合金中添加Nb,Zr,Hf元素均能够稳定β相;Zr和Hf元素的固溶强化使合金的耐磨性提高;时效处理析出α相同样使Ti-22Nb和Ti-22Nb-2Zr/Hf合金的耐磨性得到提高。
关键词:
中图分类号:TG146.2+3 文献标志码:A
Microstructure and wear behavior of biomedical Ti-Nb based alloys
WANG Ben-li1, LI Li1, ZHENG Yu-feng2
(1. Center for Biomedical Materials and Engineering, Harbin Engineering University, Harbin 150001, China;
2. College of Engineering, Peking University, Beijing 100871, China)
Abstract: The microstructure and phase constitution of biomedical Ti-22Nb, Ti-25Nb and Ti-22Nb-2Zr/Hf alloys were investigated by optical microscopy and X-ray diffractometry (XRD). The influence of the alloying elements (Zr and Hf) and heat treatment on the wear behavior of the alloys was revealed by ping-on-disc wear test and Vickers hardness tester. The wear morphology was observed by scanning electron microscopy. The wear mechanism was analyzed according to the experimental results. The results show that the addition of Nb, Zr and Hf elements can stabilize the β phase. The wear results indicate that the wear resistance of solution-treated titanium alloys can be improved because of the solid solution strengthening of Zr and Hf elements. In addition, aging process can also improve the wear resistance of the Ti-22Nb and Ti-22Nb-2Zr/Hf alloys with the precipitation of α phase.
Key words: Ti-Nb based alloys; biomedical Ti alloy; microstructure; wear behavior
钛和钛合金具有良好的力学性能、优异的抗腐蚀性和生物相容性,已成为人工关节、人工齿根等硬组织替代和修复的首选材料,特别是β钛合金以其低模量和更优异的力学相容性和生物安全性吸引了世界各国材料工作者和医学工作者的关注[1],已有各种各样的β型钛合金被开发出来,例如Ti-35Nb-5Ta-7Zr[2],Ti-12Mo-6Zr-2Fe[3],Ti-11.5Mo-6Zr-4.5Sn[4],Ti-13Nb- 13Zr[5],Ti-29Nb-13Ta-4.6Zr[6]、形状记忆β钛合金[7]、Ti-24Nb-4Zr-7.9Sn[8]和TLM[9]合金等。然而,作为硬组织替代材料,合金的耐磨性是一项重要的指标,耐磨性较差的合金在长期服役过程中会导致恶性细胞反应、组织发炎、破坏性酶的释放、骨质溶解、感染、植入物的松动和疼痛等[10]。β钛合金的耐磨性较差,人们采用各种工艺来提高合金的耐磨性,例如物理气相沉积[11]、离子注入[12]、等离子电解氧化[13]、热氧 化[14]、表面氮化[15]、激光表面改性等[15]。
本文作者采用金相组织观察、X射线衍射分析、摩擦实验、扫描电镜观察和硬度实验研究了生物医用Ti-Nb基合金(Ti-Nb,Ti-Nb-Zr及Ti-Nb-Hf)的显微组织结构和耐磨性,主要考察了合金元素(Zr、Hf)和热处理工艺对耐磨性的影响,分析了其摩擦机制。
1 实验
实验原料为0级海绵钛、纯度为99.99%(质量分数)的铌丝、锆丝和铪丝。采用高真空电弧熔铸炉熔炼设计成分的合金(合金成分均为摩尔分数),随后被冷轧成约1 mm厚的板材,变形量约95%。冷轧后的板材在900 ℃固溶0.5 h后水冷;固溶处理的合金在500 ℃时效1 h。金相组织观察使用Aiovert 200 MAT金相显微镜,浸蚀剂为40% ![]() =
= ![]() 。合金的相组成采用Philip X’Pert Pro X射线衍射仪分析,试验条件为:Cu-Kα (λ=1.540 6 nm),管电压40 kV,管电流40 mA,扫描速度2 (?)/min。耐磨性测试采用圆盘式摩擦实验机,试样固定在中间,Si3N4球形压头绕试样转动,转速为120 r/min,压下载荷为0.5 N。采用扫描电镜观察摩擦后的表面形貌。采用维氏硬度计进行显微硬度测试。
。合金的相组成采用Philip X’Pert Pro X射线衍射仪分析,试验条件为:Cu-Kα (λ=1.540 6 nm),管电压40 kV,管电流40 mA,扫描速度2 (?)/min。耐磨性测试采用圆盘式摩擦实验机,试样固定在中间,Si3N4球形压头绕试样转动,转速为120 r/min,压下载荷为0.5 N。采用扫描电镜观察摩擦后的表面形貌。采用维氏硬度计进行显微硬度测试。
2 结果与讨论
图1所示为固溶态Ti-22Nb和Ti-25Nb合金的光学显微组织。从图1可以看出,Ti-22Nb合金固溶后晶粒呈多边形,晶界平直,晶粒内部观察到取向不同的马氏体板条,合金由α″马氏体相和β相组成。Ti-25Nb合金固溶后晶粒大小不一,晶界明显,没有观察到板条状马氏体。固溶态合金在500 ℃时效1 h后,Ti-22Nb合金由α相和β相组成,Ti-25Nb合金没有观察到明显的α相衍射峰(如图2所示)。金相显微组织和XRD结果表明:添加Nb元素能够增强钛合金β相的稳定性,阻碍淬火过程中α″相的形成和时效过程中α相的析出。
Ti-22Nb-2Zr合金固溶处理后的光学显微组织如图3(a)所示,合金从高温淬火后,β相被保留下来,其光学显微组织为单相β相,β晶粒明显,晶界清晰可见。图3(b)所示为固溶处理后的Ti-22Nb-2Zr合金在500 ℃时效1 h后的光学显微组织形貌,亚稳β相分解为初生α相和转变β相。Zr和Hf与Ti属于同一族元素,向Ti-22Nb合金中添加2% 的Hf,其固溶态和时效态合金的光学显微组织与Ti-22Nb-2Zr合金类似。与图1(a)对比可以看出,添加Zr或Hf元素能够稳定β相,阻碍淬火过程中α″马氏体的析出。

图1 固溶态Ti-Nb合金的光学显微组织照片
Fig.1 Optical micrographs of solution-treated Ti-Nb alloys: (a) Ti-22Nb; (b) Ti-25Nb

图2 Ti-Nb合金的XRD谱
Fig.2 XRD patterns of Ti-Nb alloys: (a) As-aged; (b) Solution-treated
图4所示为Ti-22Nb和Ti-25Nb合金固溶和时效

图3 Ti-22Nb-2Zr合金光学显微组织照片
Fig.3 Optical micrographs of Ti-22Nb-2Zr alloy: (a) Solution-treated; (b) As-aged
处理后与Si3N4球对磨时摩擦因数随磨损时间的变化规律。从图4可以看出,在实验开始阶段,所有试样的摩擦因数随实验时间的延长而急剧上升;摩擦因数达到最大值后,固溶态Ti-Nb合金的摩擦因数在随后的整个实验时间范围内不稳定,摩擦因数波动明显且数值较大;而时效态Ti-22Nb和Ti-25Nb合金在摩擦因数达到最大值后很稳定,与固溶态合金相比,摩擦因数很小,约为0.1。
然而,固溶态Ti-22Nb-2Zr/Hf合金与固溶态Ti-22Nb合金相比,其摩擦因数的变化规律明显不同(如图5所示)。固溶态Ti-22Nb-2Zr/Hf与Si3N4球对磨时,当摩擦因数达到最大值后,摩擦因数在整个实验时间范围内比较稳定,且数值较小。与固溶态合金相比时效处理的Ti-22Nb-2Zr/Hf合金的摩擦因数变化不大。
图6~8所示为合金磨损表面的SEM像。从图6和图7可以看出:固溶态Ti-22Nb和Ti-25Nb合金磨损表面的犁沟较深;Ti-Nb合金时效后表面犁沟较浅且磨屑较多,磨损表面发生了磨屑的粘连现象。从图8可以看出:与固溶态Ti-22Nb合金摩擦后的表面不同,固溶态Ti-22Nb-2Zr/Hf合金磨损表面的犁沟较浅,合金表面分布着粘连的磨屑;时效态合金磨损表面没有发现犁沟且磨屑很少,这里只给出了Ti-22Nb-2Hf合金磨损后的表面形貌。
表1给出了实验材料在不同热处理后的维氏硬度测试结果。对于固溶态和时效态Ti-Nb合金及固溶态Ti-22Nb-2Zr/Hf合金,在与Si3N4陶瓷球对摩擦过程中磨屑首先形成,部分磨屑被黏附到陶瓷球上,使试样表面磨损,形成大量的凹坑,黏附在陶瓷球上的合金经过反复转移和挤压等发生了加工硬化、疲劳及氧化等,脱落形成游离的磨屑,磨屑在后续的摩擦过程中对磨损表面进行犁削而产生犁沟,其摩擦机制为黏着磨损。固溶态Ti-22Nb合金由α″马氏体相和β相组成,Ti-25Nb合金为单相β相,时效处理后析出α相合金的硬度增大,时效态Ti-Nb合金摩损表面犁沟较浅。添加Zr或Hf元素后,合金元素起到固溶强化的作用,Ti-22Nb-2Zr/Hf合金的硬度比二元合金的硬度高,磨损表面产生了较小的塑性变形,犁沟较浅。时效后的Ti-22Nb-2Zr和Ti-22Nb-2Hf合金硬度很高,其摩擦机制主要以磨粒磨损为主,磨损表面犁沟不明显。

图4 Ti-Nb合金摩擦因数随时间的变化曲线
Fig.4 Coefficient of friction vs time for Ti-Nb alloys using Si3N4 counter face ball: (a) Ti-22Nb; (b) Ti-25Nb
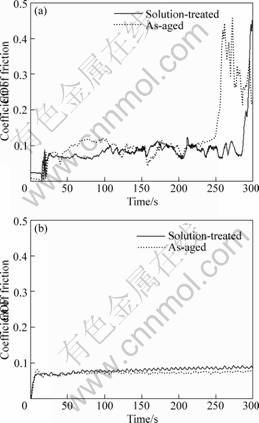
图5 Ti-22Nb-2Zr/Hf合金摩擦因数随时间的变化曲线
Fig.5 Coefficient of friction vs time for Ti-22Nb-2Zr/Hf alloys using Si3N4 counter face ball: (a) Ti-22Nb-2Zr; (b) Ti-22Nb-2Hf

图6 Ti-22Nb合金的磨痕形貌
Fig.6 Morphologies of wear surfaces of Ti-22Nb alloys: (a) Solution-treated; (b) As-aged

图7 Ti-25Nb合金的磨痕形貌
Fig.7 Morphologies of wear surfaces of Ti-25Nb alloys: (a) Solution-treated; (b) As-aged

图8 Ti-22Nb-2Hf合金的磨痕形貌
Fig.8 Morphologies of wear surfaces of Ti-22Nb-2hf alloys: (a) Solution-treated; (b) As-aged
表1 实验材料的维氏硬度
Table 1 Vickers hardness of test materials

3 结论
1) Nb,Zr和Hf元素均能够增强钛合金β相的稳定性,阻碍淬火过程中α″相的形成。
2) 与固溶态Ti-Nb合金相比,添加微量Zr或Hf的Ti-22Nb-2Zr/Hf合金经固溶处理后耐磨性有很大提高;且所有合金时效处理后耐磨性均得到提高。
3) 固溶态和时效态Ti-Nb合金及固溶态Ti-22Nb-2Zr/Hf合金的摩擦机制为黏着磨损,摩擦表面存在犁沟;时效态Ti-22Nb-2Zr/Hf合金摩擦表面犁沟不明显,摩擦机制以磨粒磨损为主。
4) 与Ti-Nb合金相比,Ti-22Nb-2Zr/Hf固溶或时效后具有较高的硬度,使其耐磨性提高。
REFERENCES
[1] GEETHA M, SINGH A K, ASOKAMANI R, GOGIA A K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review[J]. Prog Mater Sci, 2009, 54(3): 397-425.
[2] LONG M, RACK H J. Surface deformation and mcirocrack formation in Ti-35Nb-8Zr-5Ta-O(x) alloys during reciprocating sliding wear[J]. Mat Sci Eng C, 2005, 25(3): 382-388.
[3] MURRAY N G D, JABLOKOV V R, FREESE H L. Mechanical and physical properties of Ti-12Mo-6Zr-2Fe b titanium alloy[J]. ASTM Special Technical Publication, 2006, 1471: 3-15.
[4] LAHEURTE P, EBERHARDT A, PHILIPPE M J. Influence of the microstructure on the pseudoelasticity of a metastable b titanium alloy[J]. Mat Sci Eng A, 2005, 396(1/2): 223-230.
[5] LONG M, RACK H J. Titanium alloys in total joint replacement—A materials science perspective[J]. Biomaterials, 1998, 19(18): 1621-1639.
[7] YONEYAMA T, MIYAZAKI S. Shape memory alloys for biomedical applications[M]. Cambridge: Woodhead, 2009: 69-85.
[8] HAO Y L, LI S J, SUN B B, SUI M L, YANG R. Ductile titanium alloy with low Poisson’s ratio[J]. Phys Rev Lett, 2007, 98(21): 210465.
[9] YU Z, ZHOU L, FANG M, YUAN S. Investigation on near-β titanium alloy Ti-5Zr-3Sn-5Mo-15Nb for surgical implant materials[J]. Materials Science Forum, 2010, 475/479(III): 2353-2358.
[10] TORGERSON S, GJERDET N R. Retrieval study of stainless steel and titanium miniplates and screws used in maxillofacial surgery[J]. J Mater Sci Mater Med, 1994, 5(5): 256-262.
[11] PROBST J, GBURECK U, THULL R. Binary nitride and oxynitride PVD coatings on titanium for biomedical applications[J]. Surf Coat Tech, 2001, 148(2/3): 226-233.
[12] RAUTRAY T R, NARAYANAN R, KWON T Y, KIM K H. Surface modification of titanium and titanium alloys by ion implantation[J]. J Biomed Mater Res B, 2010, 93(2): 581-591.
[13] CESCHINI L, LANZONI E, MARTINI C, PRANDSTRALLER D, SAMBOGNA G. Comparison of dry sliding friction and wear of Ti6Al4V alloy treated by plasma electrolytic oxidation and PVD coating[J]. Wear, 2008, 264(1/2): 86-95.
[14] DONG H, BELL T. Enhanced wear resistance of titanium surfaces by a new thermal oxidation treatment[J]. Wear, 2000, 238(2): 131-137.
[15] TIAN Y S, CHEN C Z, WANG D Y, LEI T G. Laser surface modification of titanium alloys—A review[J]. Surf Rev Lett, 2005, 12(1): 123-130.
(编辑 赵 俊)
基金项目:中央高校基本科研业务费专项资金资助项目(HEUCFR1020)
通信作者:王本力,讲师;电话:0451-82518173;E-mail:blwang@hrbeu.edu.cn


