
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 206-214
Extraction of cerium(IV) using tributyl phosphate impregnated resin from nitric acid medium
O. S. HELALY1, M. S. ABD EL-GHANY1, M. I. MOUSTAFA1,
A. H. ABUZAID1, N. M. ABD EL-MONEM2, I. M. ISMAIL2
1. Nuclear Materials Authority, P.O.Box 530 El-Maadi, Cairo, Egypt;
2. Faculty of Engineering, Chem. Eng. Dept., Cairo University, Giza, Egypt
Received 17 March 2011; accepted 21 July 2011
Abstract:
Tributyl phosphate (TBP) solvent was used for impregnation into Amberlite XAD-16 nonionic polymeric resin beads using the wet method to prepare solvent impregnated resin (SIR). Undiluted TBP in a ratio to the resin support (volume to mass) of 6.0 at room temperature (RT) in 24 h was impregnated the resin with a mass ratio of 1.944, while the prepared gross sample of SIR at the ratio of solvent to resin of 3.0 was impregnated with a mass ratio of 1.88. Cerium(IV) oxide concentrate, prepared from crude Egyptian monazite sand, containing 37% cerium, 1.6% thorium and about 40% the other trivalent rare earth oxides, was used to prepare cerium(IV) nitrate solution for extraction using the prepared SIR. The impregnated resin was satisfactory for Ce(IV) extraction from nitric acid medium at room temperature. Cerium loading capacity of the impregnated resin reached 95.6% of the calculated theoretical capacity (173 g/kg (Ce/SIR)) under the conditions of 51.57 g/L cerium and 2.48 g/L thorium, 5.0 mol/L free nitric acid, solution to resin ratio of 10.0 and contacting the phases for 5.0 min. The loading capacity reached 98.75% when cerium concentration was increased to 91.43 g/L under the same conditions.
Key words:
cerium (IV); crude monazite sand; tributyl phosphate; impregnated resin; extraction; nitric acid medium;
1 Introduction
Abundance of cerium makes it one of the cheapest rare earth metals; however, monazite and bastnasite minerals are the world supply or both rare earth elements and thorium. Monazite sand was obtained as a by- product concentrate during the successive concentration of different economic minerals contained in the black sand deposits through physical processing and separation techniques [1].
Separation of valuable or economic elements from aqueous solutions has gained an increasing importance through selective extraction using ion exchange resins or organic solvents. In spite of solvent extraction offering fast mass transfer rate, high distribution and selectivity, it suffers from crud formation, phase disengagement and appreciable solubility and entrainment of the solvent in the aqueous phase. On the other hand, ion exchange process offers greater simplicity in equipment and operation, which enables it to economically treat complicated problems but suffer from lower selectivity and mass transfer rate than the solvent extraction [2].
The great advance in recent years drives towards bridging the gaps between ion exchange resin and solvent extraction systems through development of new effective ion selective exchanger media based on the solvent extraction, so called solvent impregnated resin “SIR”. This medium is considered a technological alternative for the extraction systems. The basic concept of solvent impregnated resins is based on simple immobilization of the most commercially used selective solvents into an inert insoluble support, especially various types of nonfunctional polymeric macroporous resin beads, such as Amberlite-XAD series which have higher efficiency than the other different supports such as silica gel, kieselguhr, and activated carbons [3-5].
The solvent was retained on the internal surface of hydrophobic nonionic resins (such as Amberlite XAD-16) by adsorption in the polymeric resin structure rather than chemical bonding [4, 6]. It has been recognized that the impregnated solvent can exhibit strong affinity for the polymeric resin matrix but still behaves as its presence in the liquid state [7, 8]. However, the impregnation of resin with a solvent is independent of the particles size of the polymeric resin support beads or the impregnation method [9, 10].
Tributyl phosphate (TBP) was the most selective solvent used commercially which also resists the strong oxidation action, so it is satisfactory for cerium (IV) extraction from nitrate aqueous solution media. Accordingly, highly pure cerium can be separated from the other trivalent rare earth, thorium and uranium, depending upon the difference in the chemical properties during cerium extraction and back-extraction [11].
The tributyl phosphate impregnated resin shows a lower solubility in water as well as in nitrate solutions than in the liquid state. In this regard, the solubility of liquid TBP in demineralized water is 0.38 g/L while its solubility in a polymeric resin support varies from undetectable amount (0.02 g/L) to 0.2 g/L. Losses of the solvent from the impregnated resins are negligible in relatively concentrated salt solutions, particularly in the acidic range [12-14].
Generally, extraction using the solvent TBP requires neither chloride nor sulfate media. This is due to the fact that sulfate ions have an adverse effect upon the extraction of cerium and thorium. Besides, dissolution of cerium hydrous oxides cake in sulfuric acid is associated with partial reduction of cerium from its tetravalent state. Hydrochloric acid has also effect as a reducing agent on the cerium with additional disadvantage of the liberation of hazardous quantities of chlorine [15]. On the other hand, the extraction using TBP proceeds satisfactorily from nitric acid medium, where cerium does not change its oxidation state during the dissolution of the oxides cake. Moreover, nitric acid has actually desirable enhancing effects upon the extraction of cerium and thorium in addition adverse inhibiting extraction effect upon the other trivalent rare earth elements [13].
Cerium extraction mechanism as its tetravalent nitrate complex from an aqueous solution using the tributyl phosphate solvent (or TBP impregnated resin) proceeds according to the following equation [7, 8, 16]:
(Ce4++4NO3-)aq+2(TBP)org=[Ce(NO3)4·2TBP]org
Regarding thorium, it is mainly extracted as [Th(NO3)4·2TBP] from low free nitric acid normality with TBP, according to the following equation:
(Th4++4NO3-)aq+2(TBP)org=[Th(NO3)4·2TBP]org
While extraction of thorium from high free nitric acid normality, 3-4 moles of TBP to 1.0 mole of thorium, may be present in the complex [17]. However, high concentration of nitrate radical in the aqueous solution drives the above reactions to the right.
2 Experimental
2.1 Instruments
UV-spectrophotometer, multi positions, single beam model SP-8001 (Metretech Inc. version 1.02, 2000/10/01), with glass cell of 10 mm was used for determination of cerium and thorium.
Samples from the solvent before and after impregnation were analyzed against its TBP concentration through titration with 0.2 mol/L NaOH using 0.1% (m/v) bromthymol blue as indicator [18].
Cerium was determined (after oxidation using ammonium persulphate) [19] and thorium was determined using thoron [19].
This work involves preparation of TBP impregnated Amberlite XAD-16 polymeric resin and extraction of cerium (IV) from nitrate medium using solvent impregnated resin.
2.2 Materials and reagents
Tributyl phosphate (TBP) solvent was used in this work for impregnation onto the nonionic polymeric resin adsorbent Amberlite XAD-16 using the wet method. Before the impregnation both of the polymeric resin support and the solvent were pretreated to remove any inorganic impurities or monomeric materials from the resin beads. This was required to insure an active surface of the resin beads and to remove the hydrolyzed harmful products from the solvent, viz, dibutyl and monobutyl phosphates. In this regard, the Amberlite XAD-16 (100 g) was transferred into a glass funnel with filter paper, wetted with distilled water then washed with 1.0 L 2.0 mol/L HNO3 then 2.0 mol/L NaOH solutions. Successive washing with distilled water between the acid and soda treatments was done and finally washed with acetone then dried in an oven at 50 °C for 48 h [20].
With respect to tributyl phosphate solvent, 300 mL solvent was firstly contacted four times with equal volume of 1.5 mol/L HNO3 (in 1 L glass separating funnel) then with 1.0 mol/L NaOH solutions. Washing with distilled water between the acid and soda treatments was conducted till the neutralization point [21]. However, it is advisable, before using the pretreated solvent for impregnation, to saturate with nitric acid. Accordingly, tributyl phosphate solvent was saturated using 8.0 mol/L nitric acid where the solvent contacted three times with the acid for 2.0 min.
Cerium (IV) oxide concentrate was prepared from the crude Egyptian monazite sand graded about 47% through digestion of this monazite sand using sulphuric acid followed by leaching, precipitation of the major sodium light rare earth double sulphates, conversion to hydroxides and finally cerium oxidation steps. This concentrate contains 37% cerium, 1.6% thorium and about 40% the other trivalent rare earths respectively.
2.3 Solvent impregnation experiments
Wet impregnation method was chosen for conducting the impregnation of tributyl phosphate solvent onto the Amberlite XAD-16 polymeric resin support. The experiments were performed in 100 mL glass beaker using the pretreated TBP (diluted in kerosene) and 5.0 g the pretreated dry polymeric resin. To verify the maximum impregnation, the major factors affecting the solvent impregnation were studied at room temperature (RT). These factors involve: 1) solvent concentrations from 20% to 80% and also undiluted TBP where S/R ratio (v/m) was 6.0 and the impregnation time was 24 h; 2) solvent to polymeric resin S/R ratios from 3.0 to 6.0 where the solvent concentration was 80% TBP in kerosene and impregnated for 24 h; 3) phases contact time from 1.0 to 48 h where the solvent concentration of 80% TBP in kerosene was also used at S/R ratio (v/m) of 6.0.
After each impregnation experiment, the remained TBP solvent was separated from the impregnated resin using plastic net and its volume was determined. Samples from the solvent before and after impregnation were analyzed against its TBP concentration.
The impregnated resin samples were well washed with distilled water to free the resin beads from any adhered solvent and diluent which can be assured by disappearance of the solvent spots floating on the surface of the wash water. The washed impregnated resin samples were then dried over night in an oven at 50 °C. The dry mass was accurately determined then actual mass of the impregnated TBP was calculated. The mass of impregnated TBP relative to the mass of dry polymeric resin sample (m(TBP)/m(Resin)) was calculated according to the following equation: Impregnated TBP mass ratio=Mass of TBP impregnated/ Mass of dry polymeric resin
2.4 Experiments of cerium extraction using TBP impregnated resin
The factors generally affecting cerium extraction from nitric acid medium using TBP impregnated resin (SIR) were studied. To overcome the change in nitric acid free normality during the extraction, the TBP impregnated resin was firstly saturated with 8.0 mol/L nitric acid. Dry sample of this SIR resin (5.0 g equivalent to 8.0 mL wet settled resin “w.s.r”) was used for each experiment where the phases were agitated in a glass beaker using magnetic stirrer at room temperature. After each extraction experiment, the solution was separated from the resin by filtration using Whatman filter paper No. 42.
The series of experiments were conducted under the conditions of 51.57 and 2.48 g/L initial concentrations of cerium and thorium respectively in nitric acid medium of 5.0 mol/L free normality and solution to resin (v/m) ratio of 2.0 where the phases were agitated for 5.0 min. The ranges of these factors were as follows, where one factor was varied and the others were fixed at the pre-mentioned values: phases contact time from 1.0 to 30 min; free nitric acid concentration from 4.0 to 10 mol/L; initial cerium concentrations from 20 to 60 g/L and the corresponding thorium from 0.96 to 2.88 g/L; solution to resin S/R ratio (v/m) from 2.0 to 6.0 (for the resin loading capacity the ratio was 10.0 and the cerium concentration was 91.43 g/L); salting-out agent addition using two types, namely, ammonium and aluminum nitrates salts with nitric acid to adjust the free nitrate concentration from 4.0 to 5.0 mol/L; cerium bleeding though five successive contacts for the solution with fresh TBP impregnated resin for each contact where S/R ratio was 10.0.
Cerium and thorium extraction efficiencies were calculated according to the following equation:
![]() (1)
(1)
where x is the extraction efficiency; ρo is the original concentration; ρr is the remaining concentration.
The saturation capacity of TBP impregnated resin (SIR) for cerium was calculated according to the following equation:
![]() (2)
(2)
where Q is the SIR saturation capacity; a1 is the actual loaded cerium; q0 is theoretical loading capacity.
3 Results and discussion
3.1 Results of solvent impregnation of Amberlite XAD-16
The results of studying factors affecting the impregnation will be herein discussed which involve impregnated solvent concentration, contact time and the solvent to polymeric resin ratio.
3.1.1 Effect of tributyl phosphate concentration
The results of effect of solvent concentration on impregnating of Amberlite XAD-16 (Fig. 1) reveal that the impregnated solvent was gradually increased from 0.436 to 1.728 g/g (TBP/resin) by increasing the concentration of TBP solvent from 20% to 80%. The maximum impregnation for the resin reached 1.944 g (TBP/resin) when the undiluted TBP was used. The maximum impregnated amount of solvent is higher by 35% than that impregnated using Amberlite XAD-4 which was only 1.26 g/g [22]. However, it was found that with increasing the solvent concentration on the impregnated resin, the extraction efficiency was also increased [23].

Fig. 1 Effect of TBP concentration on Amberlite XAD-16 impregnation
With regard to the solvent concentration after the impregnation, it is obvious from Table 1 that TBP concentration was increased by 3%-4% from the tested concentration range of 20%-80%. This increasing may result from more adsorption amount of the diluent kerosene.
Table 1 TBP concentration before and after impregnation

3.1.2 Effect of impregnation contact time
The effect of contact time on TBP impregnation onto Amberlite XAD-16 polymeric resin was studied to attain the minimum time required for a maximum impregnation. The results of this factor are illustrated in Fig. 2. From the results it is clear that the impregnation of TBP solvent proceeds rapidly from the first hour where it reached 1.284 g/g and increased steadily to 1.728 g/g after 24 h. After 48 h, TBP impregnation reached 1.864 g/g, which is less by about 4.0% than that in the case that undiluted TBP is used for impregnation period of 24 h.
Taking in consideration of the solvent concentration after the impregnation, it is obvious from Table 2 that the concentration of TBP was slightly decreased after 1 h while the concentration was gradually increased to 81.20% after 3 h and to 83.76% and 84.10% after impregnation for 24 h and 48 h, respectively. This reveals that the diluent kerosene was adsorbed appreciably by the polymeric resin when the impregnation time was increased.

Fig. 2 Effect of contact time on TBP/Amberlite XAD-16 impregnation
Table 2 TBP concentration before and after impregnation

3.1.3 Effect of TBP/Amberlite XAD-16 ratio
The effect of TBP/Amberlite XAD-16 ratio on impregnation was studied to identify the suitable ratio. The results are illustrated in Fig. 3. From the results it is obvious that the amount of TBP impregnated slightly increased from 1.326 to 1.4 and 1.506 g/g when the ratio was increased from 3.0 to 4.0 and 5.0 respectively.

Fig. 3 Effect of TBP/polymeric resin ratio on impregnation
Increasing the ratio to 6.0, the amount of TBP impregnated was increased to 1.766 g/g resin.
It is worthy to mention that the TBP/polymeric resin ratio of 3.0 is the minimum ratio to just cover the surface of the Amberlite XAD-16 polymeric resin support. This is also reflected from that at the ratio of 6.0 about half of the solvent volume was separated after the impregnation. On the other hand, the solvent concentration after the impregnation (Table 3) was substantially increased to 85.82% at the ratio of 3.0 and was gradually decreased to 83.76% when the ratio was increased to 6.0. This is as previously mentioned due to adsorbing more amounts of kerosene which reflects the higher concentrations of TBP at the lower ratios.
Table 3 TBP concentration before and after impregnation

In conclusion, the suitable conditions for the maximum impregnation of the tributyl phosphate solvent onto the Amberlite XAD-16 polymeric resin support were undiluted solvent to resin ratio of 3.0 and impregnation time of 24 h. Accordingly, the gross TBP impregnated resin sample was prepared with 1.88 g (TBP/polymeric resin). This prepared sample was used in the further experiments of cerium extraction from nitrate medium.
3.2 Results of cerium extraction using TBP impregnated resin
The results of studying the factors affecting cerium/thorium extraction using TBP impregnated Amberlite XAD-16 resin from nitric acid medium would be herein discussed. These factors involved extraction contact time, free nitric acid concentration, initial cerium/thorium concentrations, salting-out agents addition, solution to resin ratio (also resin loading capacity) and finally cerium bleeding from the solution.
3.2.1 Extraction contact time
The results of studying this factor using the TBP impregnated resin are illustrated in Fig. 4. From the results it is obvious that cerium extraction was proceeding rapidly from the first minute of contact (48%) and reached its maximum extraction efficiency of 67.8% after 5.0 min. Increasing the contact time more than 5.0 min, the extraction efficiency would be dramatically decreased to as low as 44.5% after 30 min. This decrease
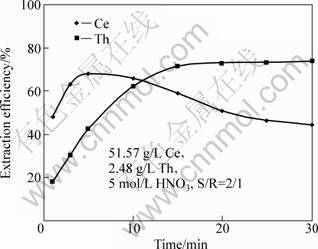
Fig. 4 Effect of contact time on cerium and thorium extraction efficiency
may be due to the beginning of extraction of trivalent rare earths which replace the extracted cerium.
Regarding to the thorium extraction, the results reveal that its extraction efficiency was increased steadily, where it was 18.2% at the first minute and 73.6% after 30 min. Accordingly, contacting the phases for 5.0 min would be enough at which extraction efficiencies of 67.8% and 42.4% for cerium and thorium were achieved respectively.
3.2.2 Free nitric acid concentration
The results of studying this factor are indicated in Fig. 5 for cerium and Fig. 6 for thorium. The results reveal that the free nitric acid concentration of 5.0 mol/L verified the maximum cerium extraction efficiency of 67.8% after 5.0 min of contact time, but this efficiency was not increased excess 44.5% after 30 min. Increasing the free concentration more than 5.0 mol/L, the extraction efficiency would be decreased dramatically to as low as 36.1% and 11.3% at 10 mol/L free nitric acid after 5.0 and 30 min, respectively. It is worthy to mention that, the presence of free nitric acid in the solution actually increases cerium extraction efficiency due to the salting-out effect, which enhances the extraction. It was found that the maximum cerium extraction efficiency is achieved at a free nitric acid concentration varying from 4.0 to 5.0 mol/L. However, the increase in free nitric acid concentration leads to a competitive effect of the acid with the required extracted complexes by TBP, which would thus lead to lower cerium extraction efficiency [24].

Fig. 5 Effect of free nitric acid concentration on cerium extraction efficiency

Fig. 6 Effect of free nitric acid concentration on thorium extraction efficiency
With respect to the thorium extraction efficiency, it was clear that increasing the free nitric acid concentration enhances greatly the extraction efficiency to 90% at a free nitric acid of 10 mol/L after 5.0 min of contact time and after 30 min extraction has almost completed at the same free nitric acid concentration. This result reflects that at high free nitric acid concentration (more than 6.0 mol/L) thorium forms nitrate complexes which are extracted more easily than cerium [17].
3.2.3 Initial cerium/thorium concentration
The results of studying this factor are illustrated in Figs. 7 and 8 for cerium and thorium, respectively. The results show that cerium extraction efficiency was gradually increased from 71.25% to 81.5% by increasing the initial cerium concentration from 20 g/L to 40 g/L. Increasing the concentration to 50-60 g/L, the extraction efficiency would be decreased by 10%-12% to 69.3% at the concentration 60 g/L. However, the extraction efficiency of thorium was increased from 20.2% to 46.2% by increasing its concentration from 0.96 to 2.88 g/L.
Actually, the extracted amount of cerium (Table 4) was greatly increased where it was 28.5 to 83 g/kg at the concentrations of 20 and 60 g/L, respectively, while the co- extracted amounts of thorium were 0.38 and 2.66 g/kg. However, the percent of extracted thorium/cerium was decreased by 0.7% ((1.30×3-3.2)%=0.7%) when cerium concentration was increased from 20 to 60 g/L.

Fig. 7 Effect of initial cerium concentration on extraction efficiency

Fig. 8 Effect of initial thorium concentration on extraction efficiency
Table 4 Extracted amounts of cerium and thorium with different concentrations

3.2.4 Salting-out agent addition
The results of studying salting-out agent addition, namely, ammonium and aluminum nitrates, are illustrated in Table 5. The results show that cerium extraction efficiency was enhanced when either of the salting-out agents was added and greatly enhanced by increasing their concentrations.
Table 5 Effect of salting out agent addition on cerium extraction efficiency

In this regard, at a total free nitrate of 4.0 mol/L, the extraction efficiency was increased from 70.3% to 80.1% when NH4NO3 concentration was increased from 1.0 to 2.0 mol/L and the efficiency was increased from 71.7% to 78.2% when Al(NO3)3 was used as a salting-out agent. In a total free nitrate of 5.0 mol/L, the extraction efficiency was increased from 72.4% to 80.6% for NH4NO3 concentration of 1.0 and 3.0 mol/L respectively, and the efficiency was increased from 74.0% to 81.5% in the case of the same Al(NO3)3 concentration.
It is worthy to mention that the extraction efficiencies without salting-out agent addition were only 65.4% and 67.8% at the free nitric acid concentration of 4.0 and 5.0 mol/L, respectively. However, it may be preferable to use NH4NO3 rather than Al(NO3)3 as a salting-out agent to prevent addition of foreign heavy metal; however, the difference in their extraction enhancement was near.
3.2.5 Solution to TBP impregnated resin ratio
The results of the studying solution to TBP impregnated resin (S/R) ratio are indicated in Fig. 9. The results reveal that cerium loading capacity by TBP impregnated resin (SIR) was gradually increased from 41.2% at the S/R ratio 2.0 and was 81.3% at the ratio 6.0. Increasing the solution to resin ratio to 10.0, cerium loading capacity reached 95.6% of the calculated theoretical capacity for cerium (173 g/kg).
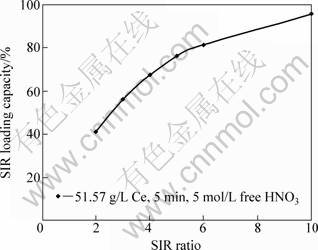
Fig. 9 Effect of solution to TBP impregnated resin ratio on loading capacity
Regarding to the conducted experiment using solution containing 91.43 g/L Ce at S/R ratio 10, the result reveals that cerium loading capacity by the TBP impregnated resin reached 98.75% of the theoretical capacity.
3.2.6 Cerium bleeding
Bleeding of cerium contained in the solution was also studied to evaluate the overall extraction. The solution to resin ratio was 10.0 and the phases contacted for 5.0 min. The results of these experiments are illustrated in Fig. 10. From the results, it is obvious that cerium concentration was decreased gradually from 51.57 g/L to 2.45 g/L after contacting five times with fresh TBP impregnated resin in each contact. However, cerium concentration was decreased by 16.54 g/L at the first contact while decreased by 8.94 g/L and 7.7 g/L after the third and the fourth contact respectively and by 5.1 g/L at the fifth contact. This is of course due to the decrease in driving force of the concentration. However, as previously mentioned the saturation capacity of TBP impregnated resin reached 95.6% (165.4 g/kg) of its theoretical saturation capacity which is equivalent to 173 g/kg.
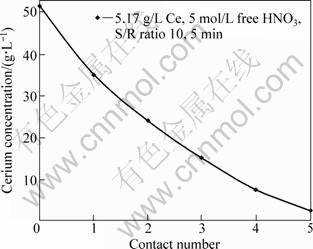
Fig. 10 Cerium bleeding from aqueous solution
4 Conclusions
1) Amberlite XAD-16 polymeric resin was impregnated at room temperature (RT) to 1.728 and 1.864 g/g (TBP/resin) after 24 and 48 h, respectively, under the conditions of TBP concentration 80% (in kerosene) and solvent/resin (S/R) (v/m) ratio 6.0. Under the same conditions, it was impregnated to a maximum level of 1.944 g/g (TBP/resin) when undiluted TBP was used for 24 h impregnation. However, the gross SIR sample was impregnated by 1.88 g/g when undiluted TBP, solvent/resin ratio 3.0 and impregnation 24 h were applied. It is worthy to mention that S/R ratio of 3.0 is the minimum ratio for just covering the resin beads surface.
2) The suitable conditions for Ce(IV) extraction from nitric acid medium using TBP impregnated Amberlite XAD-16 resin were 5.0 min contact time at room temperature (RT) and 5.0 mol/L free nitric acid concentration. The present amount of thorium does not affect the TBP impregnated resin loading capacity where the capacity reached 98.75% of the calculated theoretical capacity (173 g Ce/kg SIR). Cerium extraction efficiency was greatly enhanced when nitrate salt was added as salting-out agent with a total free nitrate concentration of 5.0 mol/L. The extraction efficiency without salting-out agent addition was 67.8% at the free nitric acid concentration of 5.0 mol/L. This efficiency was increased to 72.4% and 80.6% when 1.0 and 3.0 mol/L NH4NO3 were added, respectively.
References
[1] HEDRICK J B. Rare earths [J]. Minerals Yearbook, 2003, 60: 1-15.
[2] STREAT M, NADEN D. Ion exchange in uranium extraction [M]// Ion Exchange and Separation Processes in Hydrometallurgy, 1987.
[3] STREAT M. Ion exchange processes in hydrometallurgy [M]//Ion Exchangers. Berlin, New York: de GRUYTER, 2011: 1061-1072.
[4] SAHA B, GILL R J, BAILEY D G, KABAY N, ARDA M. Sorption of Cr(VI) from aqueous solution by Amberlite XAD-7 resin impregnated with Aliquat 336 [J]. Reactive and Functional Polymers, 2004, 60: 223-244.
[5] ABDEL RAOUF M W, EL-KAMASH A M. Kinetic and thermodynamic studies for sorption of uranium and thorium ions from nitric acid solutions onto a TBP impregnated sorbent [J]. Arab Journal of Nuclear Sciences and Applications, 2005, 38(3): 85-96.
[6] KAUCZOR H W, MEYER A. Structure and properties of Levextrel resins [J]. Hydrometallurgy, 1978, 3(1): 65-73.
[7] CORTINA J L, MIRALLES N, AGUILAR M, SASTRE A M. Solvent impregnated resins containing di-(2-ethylhexyl)phosphoric acid (I). Preparation and study of the retention and distribution of the extractant on the resin [J]. Solvent Extraction and Ion Exchange, 1994, 12(2): 349-369.
[8] CORTINA J L, MIRALLES N, SASTRE A, AGUILAR M, PROFUMO A, PESAVENTO M. Solvent impregnated resins containing di-(2, 4, 4-trimethylpentyl)phosphinic acid (II). Study of the distribution equilibria of Zn(II), Cu(II)and Cd(II) [J]. Reactive Polymers, 1993, 21(1-2): 103-116.
[9] JERABEK K, HANKOVA A, STRICKOVSKY G, WARSHAWSKY A. Solvent impregnated resins; relation between impregnation process and polymer support morphology (I). Di(2-ethylhexyl) dithiophosphoric acid [J]. Reactive and Functional Polymers, 1996, 28(2): 201-207.
[10] ROVIRA M, HURTADO L, CORTINA J L, ARNALDOS J, SASTRE A M. Recovery of palladium(II) from hydrochloric acid solutions using impregnated resins containing Alamine 336 [J]. Reactive and Functional Polymers, 1998, 38(2): 279-287.
[11] KEDARI C S, PANDIT S S, RAMANUJAM A. In situ electro-oxidation and liquid-liquid extraction of cerium(IV) from nitric acid medium using tributyl phosphate and 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate [J]. Journal of Radioanalytical and Nuclear Chemisty, 1997, 222(1-2): 141-147.
[12] KROEBEL R, MEYER A. Application of newly developed materials for extraction chromatography of inorganic salts in columns [C]// Proceeding of the International Solvent Extraction Conference, ISEC-74. London: Society of Chemical Industry, 1974: 2095.
[13] ALCOCK K, GRIMLEY S S, HEALY T V, KENNEDY J, MCKAY H A C. The extraction of nitrates by tri-n-butyl phosphate (TBP) (1). The system TBP+diluent+H2O+HNO3 [J]. Transactions of the Faraday Society, 1956, 52: 39-47.
[14] KUMAR S, KOGANTI S B. Salt effect model for aqueous solubility of TBP in a 5 to 100% TBP/n-dodecane-nitric acid-water biphasic system at 298.2 K [J]. Nuclear Technology, 2000, 129(2): 279-283.
[15] BEARSE A E, CALKINS G D, CLEGG J W, FILBERT R B. Thorium and rare earths from monazite [J]. Chemical Engineering and Processing, 1954, 50(5): 235-239.
[16] GONZALEZ-LUQUE S, STREAT M. Uranium sorption from phosphoric acid solutions using selective ion exchange resins. I. Isotherms of equilibrium extraction and desorption [J]. Hydrometallurgy, 1983, 11(2): 207-225.
[17] KOLTHOFF I M, ELVING P J, SANDELL E B. Treatise on analytical chemistry [M]. Vol. 5, II. New York: Interscience Publishers, 1961.
[18] DeSESA M A. Raw materials development laboratory handbook of analytical methods [R]. Winchester: National Lead Company, Inc., Massachusetts Information TID-7002, Rev. 1, 1957.
[19] MARCZENKO Z. Spectrophotometric determination of elements [M]. New York: John Wiley and Sons Inc, 1986: 442-443.
[20] MATSUNAGA H, ISMAIL A A, WAKUI Y, YOKOYAMA T. Extraction of rare earth elements with 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate impregnated resins having different morphology and reagent content [J]. Reactive and Functional Polymers, 2001, 49(3): 189-195.
[21] SALEH F A, FARAG A K. Recovery of uranium, thorium, and cerium from Egyptian monazite sands [J]. Zeitschrift für Anorganische und Allgemeine Chemie, 1970, 375: 308-314.
[22] KHAWASSEK Y M. Modification of the purity of the extracted material from the radioactive mineral resources [D]. Egypt: Faculty of Engineering, Alexandria University, 2008.
[23] MENDOZA R N, MEDINA T I S, VERA A, RODRIGUEZ M A. Study of the sorption of Cr(III) with XAD-2 resin impregnated with di-(2,3,4-trimethylpentyl)phosphinic acid (Cyanex 272) [J]. Solvent Extraction and Ion Exchange, 2000, 18(2): 319-343.
[24] BRAUN T, GHERSINI G. Extraction choromatography: [M]. Vol. 2. New York: Elsevier, 1975.
用磷酸三丁酯浸渍树脂从硝酸介质中提取铈(IV)
O. S. HELALY1, M. S. ABD EL-GHANY1, M. I. MOUSTAFA1,
A. H. ABUZAID1, N. M. ABD EL-MONEM2, I. M. ISMAIL2
1. Nuclear Materials Authority, P.O.Box 530 El-Maadi, Cairo, Egypt;
2. Faculty of Engineering, Chem. Eng. Dept., Cairo University, Giza, Egypt
摘 要:用磷酸三丁酯(TBP)溶剂浸渍高分子交换树脂XAD-16来制备浸渍树脂。在室温下,将未稀释的磷酸三丁酯与树脂按液固比6(磷酸三丁酯体积与树脂质量比)浸渍24 h得到的浸渍树脂的质量比为1.944;按液固比3.0浸渍24 h,得到的浸渍树脂的质量比为1.88。将从埃及独居石矿砂得到的含37%铈、1.6%钍和40%三价稀土氧化物的氧化铈(IV)精矿浸出得到的硝酸铈溶液,用所制备的浸渍树脂从该铈溶液提取铈。在室温下,用该浸渍树脂从硝酸介质中提取Ce(IV)的效果是令人满意的。在溶液中含51.57 g/L铈、2.48 g/L钍、5.0 mol/L游离硝酸,溶液与树脂比为10.0,浸渍时间为5.0 min的条件下,该浸渍树脂对Ce(IV)的负载容量达到理论值(173 g/kg (Ce/SIR))的95.6%。在其他条件不变的情况下,将铈的浓度提高到91.43 g/L,浸渍树脂对Ce(IV)的负载容量达到理论值的98.75%。
关键词:铈(IV);独居石砂;磷酸三丁酯;浸渍树脂;提取;硝酸介质
(Edited by YANG Hua)
Corresponding author: M. S. ABD EL-GHANY; Tel: 002-02-26981596; E-mail: omneya@link.net
DOI: 10.1016/S1003-6326(11)61162-X
Abstract: Tributyl phosphate (TBP) solvent was used for impregnation into Amberlite XAD-16 nonionic polymeric resin beads using the wet method to prepare solvent impregnated resin (SIR). Undiluted TBP in a ratio to the resin support (volume to mass) of 6.0 at room temperature (RT) in 24 h was impregnated the resin with a mass ratio of 1.944, while the prepared gross sample of SIR at the ratio of solvent to resin of 3.0 was impregnated with a mass ratio of 1.88. Cerium(IV) oxide concentrate, prepared from crude Egyptian monazite sand, containing 37% cerium, 1.6% thorium and about 40% the other trivalent rare earth oxides, was used to prepare cerium(IV) nitrate solution for extraction using the prepared SIR. The impregnated resin was satisfactory for Ce(IV) extraction from nitric acid medium at room temperature. Cerium loading capacity of the impregnated resin reached 95.6% of the calculated theoretical capacity (173 g/kg (Ce/SIR)) under the conditions of 51.57 g/L cerium and 2.48 g/L thorium, 5.0 mol/L free nitric acid, solution to resin ratio of 10.0 and contacting the phases for 5.0 min. The loading capacity reached 98.75% when cerium concentration was increased to 91.43 g/L under the same conditions.
[1] HEDRICK J B. Rare earths [J]. Minerals Yearbook, 2003, 60: 1-15.
Ion exchange processes in hydrometallurgy [M]//Ion Exchangers. Berlin, New York: de GRUYTER, 2011: 1061-1072." target="blank">[3] STREAT M. Ion exchange processes in hydrometallurgy [M]//Ion Exchangers. Berlin, New York: de GRUYTER, 2011: 1061-1072.
[24] BRAUN T, GHERSINI G. Extraction choromatography: [M]. Vol. 2. New York: Elsevier, 1975.


