
Electrochemical behaviors of anodic alumina sealed by
Ce-Mo in NaCl solutions
Tian Lian-peng(田连朋), Zhao Xu-hui(赵旭辉),
Zhao Jing-mao(赵景茂), Zhang Xiao-feng(张晓丰), Zuo Yu(左 禹)
School of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Received 9 November 2005; accepted 23 February 2006
Abstract:
The elimination of toxic materials in sealing methods for anodic films on 1070 aluminum alloy was studied. The new process uses chemical treatments in cerium solution and an electrochemical treatment in a molybdate solution. Potentiodynamic polarization and electrochemical impedance spectroscopy(EIS) were used to study the influences of sealing methods on the corrosion behavior of anodic films in NaCl solutions. The results show that the Ce-Mo sealing makes the surface structure and morphology of anodic films uniform and compact. Ce and Mo produce a cooperative effect to improve the corrosion resistance of anodic films. Anodic films sealed by Ce-Mo provide high corrosion resistance both in acidic and basic solutions.
Key words:
1070 aluminum alloy; anodic film; sealing; electrochemical impedance spectroscopy;
1 Introduction
Aluminum is a kind of material with active chemical behavior. It can form a thin oxide film on its surface in natural environment. The structure of this amorphous film is loose, so it could not provide good corrosion resistance. Anodizing is one of the most common methods of aluminum surface processing. Anodizing could greatly improve the corrosion resistance of aluminum, and there are many literatures on the mechanism[1-3]. The anodic film on aluminum commonly consists of two layers[4]: a thick porous outer layer separated from the metal by a thin non-porous layer is called the barrier layer; the outer layer is characterized as a close packed array of columnar hexagonal cells, each containing a central pore that is normal to the substrate surface. When the thickness of the anodic film is fixed, the corrosion resistance of the film depends on the sealing process[5]. Sealing process is necessary for the porous layer of the anodic film in order to improve the corrosion resistance of the alloy. Some sealing techniques have been commonly used in industry, such as hot water sealing, cold nickel fluoride sealing and dichromate sealing[6]. Nickel acetate sealing is widely used in North America[7]. Though there are many studies on sealing treatment[8, 9], different sealing methods improve corrosion resistance of the anodized alloys to various extends. Sealing methods containing the nickel salt and the hexavalent chromium will bring the environment great pollution. Nickel ions in the nickel salt are common contaminations, and they are carcinogenic substances. The dichromate sealing can produce toxic chemicals (Cr6+) that are poisonous for human[10]. Traditional sealing methods, which rely on toxic chemicals, can provide corrosion protection after anodizing baths. But it is an urgent affair to study the replacement of hazardous chemicals such as Cr6+ and nickel salt in corrosion protection. MANSFELD et al[11,12] reported processes using chemical treatments in various cerium salt solutions and an electrochemical treatment in a molybdate solution. It was shown that passive layers containing Ce and Mo were formed on the surface of the aluminum alloy, and their corrosion resistance had been improved by inhibition of both anodic and cathodic reaction. Local cathodes were eliminated during the surface modification process, thereby reducing the driving force for pitting. In this work, we applied the Ce-Mo sealing method to anodic films. The influences of sealing methods on the corrosion behavior of anodic films on 1070 aluminum alloy are studied by potentiodynamic polarization and electro- chemical impedance spectroscopy(EIS). The Ce-Mo sealing method is developed as a green technology for corrosion protection.
2 Experimental
2.1 Material
The materials studied were commercial purity aluminum 1070 (99.5% Al, 0.25% Fe, 0.20% Si, 0.015% Cu, mass fraction) in tempered condition.
2.2 Anodizing process
The surfaces of the alloy samples were finished using SiC abrasive paper up to 800#, and cleaned with water and acetone. A degreasing treatment was carried out for the samples in 50 g/L NaOH solution for 2 min at room temperature. After water cleaning, the samples were first chemically polished in 200 g/L HNO3 solution for 2 min at room temperature, then anodizing was carried out in 200 g/L H2SO4 solution at a current density of 2.0 A/dm2 for 30 min at 20 ℃. Then the samples were cleaned with water and dried. The thickness of the anodic film obtained was approximately 10 μm.
2.3 Sealing methods
1) Boiling water sealing: the specimens were put into boiling water (pH=6-7.5) for 30 min.
2)Dichromate sealing: the specimens were dipped in 50-70 g/L potassium dichromate solution (90-95 ℃, pH=6-7) for 30 min.
3) Nickel fluoride sealing: the specimens were first dipped in 1.2 g/L NiF2 solution (pH=5.5-6.5, t=(25±2) ℃) for 20 min, then were put into 60-70 ℃ water for 15 min.
4) Nickel acetate sealing: the specimens were put into nickel acetate solution (c(Ni2+)=1.4-1.8 g/L, pH=5.5-6.0, 85-95 ℃) for 30min.
5) Ce-Mo sealing: the specimens were first dipped in 10 mmol/L Ce(NO3)3 solution (pH=6.8-6.9, 40 ℃) for 2 h, then were put into 5 mmol/L CeCl3 solution (pH=4.5-4.8, 40 ℃) for 2 h, finally a potentiostatic polarization (+500 mV vs SCE)in 0.1 mol/L Na2MoO4 (pH=8.5) solution for 2 h was carried out.
After sealing, all the specimens were rinsed with water and dried.
2.4 Electrochemical tests and surface examination
The specimens were coated with epoxy resin, leaving an area of 1 cm2 exposed for electrochemical testing. A saturated calomel electrode(SCE) was used as the reference electrode, and the counter electrode was platinum. Polarization measurements were performed with a potentiostat-galvanostat (EG & G model 273A) and M352 corrosion measurement system in neutral (pH=7), acidic (pH=1) and basic (pH=13) 1 mol/L NaCl solutions at 25 ℃. The potential scanning rate was 1 mV/s. EIS measurement was performed at the open circuit potential with a perturbation amplitude of 10 mV and the frequency ranging from 100 kHz to 10 mHz. Each electrochemical measurement was repeated at least three times. The electrochemical results were verified by GB/T8753.1—2005 (Anodizing of aluminum and aluminum alloys—Assessment of quality of sealed anodic oxide coatings, Part 1: Phosphoric acid/chromic acid test without nitric acid predip).
The surfaces of the samples were observed using a scanning electron microscope, and the surface compositions of the films were analyzed using electron microprobe analysis.
3 Results and discussion
Polarization curves of anodized 1070 aluminum alloy sealed by different methods in 1 mol/L NaCl solutions are shown in Fig.1, Fig.2 and Fig.3. The anodic film sealed by Ce-Mo process shows low anodic current density in three kinds of solutions (lower than 10-7). In neutral 1 mol/L NaCl solution, the anodic film sealed by Ce-Mo process results in the lowest passive current densities. In acidic 1 mol/L NaCl solution, the passive current density for the film sealed by Ce-Mo process is a little higher than that of the specimen sealed by cold nickel fluoride, but no pitting occurs in the higher potential range (up to 2 V). In basic 1 mol/L NaCl solution, the anodic film sealed by dichromate shows higher anodic current density. The specimens sealed by Ce-Mo process show stable passivity, and are about two orders of magnitude lower than those sealed by hot water sealing and cold nickel fluoride.
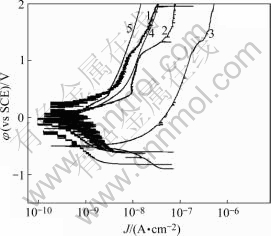
Fig.1 Polarization curves of anodized 1070 aluminum alloy sealed by different methods in neutral 1 mol/L NaCl solution (pH=7.0): 1 Boiling water sealing; 2 Nickel fluoride sealing; 3 Potassium dichromate sealing; 4 Nickel acetate sealing; 5 Ce-Mo sealing
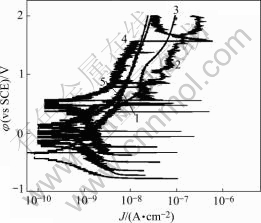
Fig.2 Polarization curves of anodized 1070 aluminum alloy sealed by different methods in acidic 1mol/L NaCl solution (pH=1.0): 1 Boiling water sealing; 2 Nickel fluoride sealing; 3 Potassium dichromate sealing; 4 Nickel acetate sealing; 5 Ce-Mo sealing.
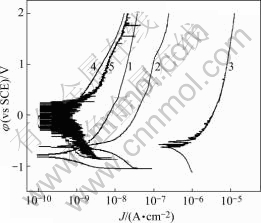
Fig.3 Polarization curves of anodized 1070 aluminum alloy sealed by different methods in basic 1mol/L NaCl solution (pH=13.0): 1 Boiling water sealing; 2 Nickel fluoride sealing; 3 Potassium dichromate sealing; 4 Nickel acetate sealing; 5 Ce-Mo sealing.
Fig 4, Fig.5 and Fig.6 are the Bode plots showing data of anodic films with different sealing processes in 1 mol/L NaCl solutions. HITZIG et al[13] have shown that the high and medium frequency portions of the impedance spectra reflect the porous layer properties of the anodic film, while the low frequency portion characterizes the barrier layer properties. Since water penetration and metal corrosion change the resistance of anodic films, the high frequency impedance modulus is particularly interesting. As the resistance of the porous layer of anodic films, higher Rp values show a more difficult penetration of the aggressive electrolyte into the anodic film on the aluminum and a better quality of the sealing method. Considering that the electrolytic resistance Rsol is very small, the Rb value is extremely high[14] and the sealing process mainly influences the outer layer of the anodic film, we will discuss the change of Cp, the capacitance of the porous layer of the anodic film, and Rp, the resistance of this layer. Fig 4, Fig.5 and Fig.6 show that the high and medium frequency impedance moduli of the anodic film sealed by Ce-Mo process are larger than those sealed by other processes in neutral 1 mol/L NaCl solution. In acidic and basic 1 mol/L NaCl solutions, the high and medium frequency impedance moduli of the anodic film sealed by Ce-Mo process are relatively large, which means the Ce-Mo process can provide better corrosion resistance.
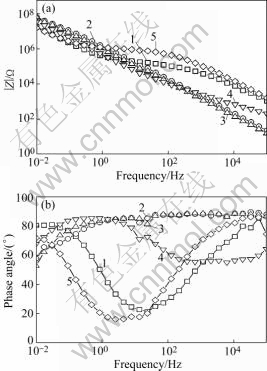
Fig.4 Bode plot showing data of anodic films with different sealing processes in neutral 1mol/L NaCl solution (pH=7.0): 1 Boiling water sealing; 2 Nickel fluoride sealing; 3 Potassium dichromate sealing; 4 Nickel acetate sealing; 5 Ce-Mo sealing
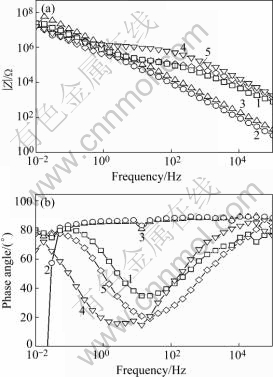
Fig.5 Bode plot showing data of anodic films with different sealing processes in acidic 1 mol/L NaCl solution (pH=1.0): 1 Boiling water sealing; 2 Nickel fluoride sealing; 3 Potassium dichromate sealing; 4 Nickel acetate sealing; 5 Ce-Mo sealing
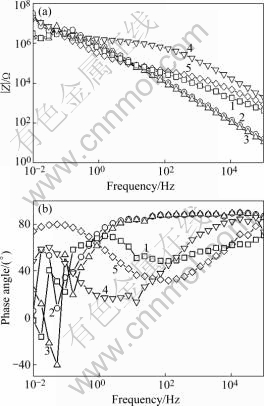
Fig.6 Bode plot showing data of anodic films with different sealing processes in basic 1 mol/L NaCl solution (pH=13.0): 1 Boiling water sealing; 2 Nickel fluoride sealing; 3 Potassium dichromate sealing; 4 Nickel acetate sealing; 5 Ce-Mo sealing
The anodic film commonly consists of two layers: a thick porous outer layer separated from the metal by a thin non-porous layer is called the barrier layer. The property of each part can be characterized by resistance and capacitance in parallel and in series to describe their electronic and dielectric behaviors. Many researchers[12-14] used different models and equivalent circuits(EC) to study the type of impedance spectra for porous anodic films on aluminum. But in the present work, only the circuit proposed by MOUTARLIER[15](Fig.7(a)) gives suitable fits. Rel is the electrolyte resistance; Rpw is the resistance of the pore walls in parallel with the capacitance Cpw. Parameters concerning the porous layer are associated to the pores and characterized by the capacitance Cp and the resistance Rp. Barrier layer properties are described by the capacitance Cb and the resistance Rb.
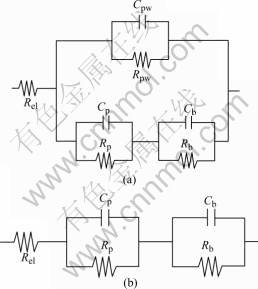
Fig.7 Equivalent circuits of impedance behavior of anodized aluminum films: (a) General model; (b) Simplified model
The resistance Rpw and the associate capacitance Cpw form one of the parallel branches in the circuit. They represent the walls of the hexagonal cells. Rpw and Cpw are generally omitted since they are extremely high and extremely low, respectively. The walls of hexagonal cells prevent the passage of current. In this way, equivalent circuit can be simplified as Fig.7(b)[16].
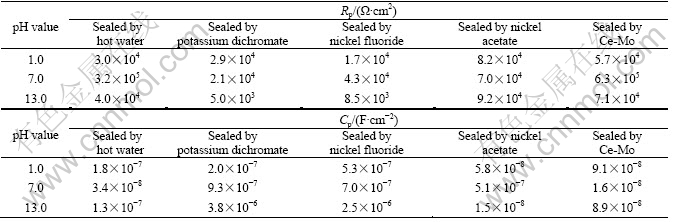
Fitting parameters for simulated spectrum to the porous layer of anodic films in 1 mol/L NaCl solution are listed in Table 1. The hydrated alumina permittivity is lower than alumina and free water permittivity. So lower Cp values indicate that there is less free water in the porous layer of anodic films and a better protectiveness of the porous layer from the corrosion solution. As the resistance of the porous layer of anodic films, higher Rp values show a more difficult penetration of the aggressive electrolyte into the anodic film on the aluminum.
Table 1 shows that in neutral NaCl solution Rp values decrease and Cp values increase in order of Ce-Mo sealing>hot water sealing>nickel acetate sealing>cold nickel fluoride sealing>dichromate sealing; in acidic NaCl solution: Nickel acetate sealing> Ce-Mo sealing>hot water sealing>dichromate sealing >cold nickel fluoride sealing; in basic NaCl solution: Nickel acetate sealing>Ce-Mo sealing>hot water sealing>cold nickel fluoride sealing>dichromate sealing. Fitting parameters for simulated spectrum are in agreement with the Bode plot data on corrosion protection.
Table 1 Fitting Parameters for simulated spectrum to porous layer of anodic films in acidic,neutral and basic l mlol/L NaCI solutions
In the hot water sealing, the majority of the hydrated aluminum is boehmite, because at temperatures above 80 ℃, the basic sealing reaction is: Al2O3+H2O→ 2AlO(OH)(boehmite). The hydrated alumina occupies a greater volume and fills the micropores in the porous anodized films. The ingress of chloride ions, water and oxygen is retarded and corrosion resistance provided by the film is improved. But boiling water sealing mainly results in physical filling of the pores, which has only limited improving effect on corrosion resistance of the anodic film in acidic and basic solutions.
The mechanism of cold nickel fluoride sealing is that fluoride ions enter the porous film and change the electric property of the anodic film[9]. Fluoride ions act as an accelerator, forming aluminum fluoride complex that may finally plug or block the pores. Because the sealing reaction in the pores of the anodic film still occurs even the chemical reactions on the surface are ceased, aging process is commonly necessary after the cold nickel fluoride sealing. Alumina is mainly converted to aluminum hydroxide since boehmite can only be formed at above 80 ℃. Ni(OH)2 deposit is formed due to hydrolysis of Ni2+ ions. F- ions react with alumina and AlF3 is also formed. The reaction products of cold nickel fluoride sealing contain Al(OH)3, Ni(OH)2 and NiF3. These products co-precipitate within the micropores of anodic films and block the pores. In acidic solution Ni(OH)2 is not stable. The dissolution of Ni(OH)2 may result in imperfections in the film, leading to susceptibility to pitting in acidic solution.
For the dichromate sealing, the bichromate will react with the alumina in the strong oxidative solution at temperatures above 90 ℃. The following reaction is present[6]:
2Al2O3+3K2Cr2O7+5H2O=2AlOHCrO4+2AlOH+Cr2O7+6KOH
The aluminum oxydichromate and aluminum oxychromate will block the micropores. The aluminum oxydichromate layer performs as a barrier layer to improve corrosion resistance. In addition, the residual hexavalent chromium provides a continuous time-release source of inhibitor for repairing the film at defect sites.
The mechanism of nickel acetate sealing is relatively more complicated[7]. Because the sealing temperature is above 80 ℃, the aluminum and water will be chemically combined into boehmite; in the mean time, nickel hydroxide is produced: Ni2++2OH-→Ni(OH)2. The co-deposition of boehmite and nickel hydroxide will block the porous layer of the anodic film better. Ni(OH)2 can be regarded as the catalyzer for producing boehmite more effectively. Boehmite and Ni(OH)2 have synergistic effect on improving corrosion resistance, so the anodic film sealed by nickel acetate has higher corrosion resistance.
Fig.8(a) shows the SEM image of the anodic film sealed by Ce-Mo process. It shows that the surface of the anodic film sealed by Ce-Mo process is uniform. Fig.8(b) shows the SEM image of the anodic film sealed by Ce-Mo process. From left to right in turn are the aluminum matrix, the anodic film and the epoxy resin. It shows that the thickness of the anodic film obtained is approximately 10 μm and no defects are observed. EMA result shows that there are O, Al, S, Mo and Ce on the surface of the anodic film sealed by Ce-Mo process. O and Al are the main components of the anodic film. S element comes from the sulfuric acid electrolyte during the anodizing.

Fig.8 SEM images of anodic film sealed by Ce-Mo process: (a) Surface; (b) Profile
The composition change of Mo and Ce with depth of the anodic films is shown in Fig.9. This result indicates that Ce and Mo exist throughout the anodic film. The peak concentrations of element Mo and Ce in the sample are at the surface of the anodic film. The Ce concentration decreases as the depth increases, but Mo concentration increases with depth. Therefore, Mo and Ce form partial components of the porous film, which should be a reason for better corrosion resistance of samples sealed by Ce-Mo process.
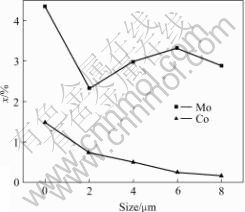
Fig.9 EMA profile analysis of anodic film sealed by Ce-Mo process
The mechanism of Ce-Mo sealing might be that Ce(NO3)3 solution and CeCl3 solution firstly modify the surface of the anodic film, then cerium salt deposits to chemically block the porous film. During the electrochemical process, ![]() enters the porous film to restrain corrosion, which is similar to Cr6+ in dichromate sealing. The incorporation of Ce and Mo in the outer porous layer of the anodic film reduces the susceptibility to local corrosion. During the anodizing, there are many sites where metal precipitates are located. Mo and Ce concentrate at these sites to reduce the extent and activity of local cathodes. In Ce-Mo sealing, Ce and Mo produce a cooperative effect to improve the corrosion resistance of anodic films.
enters the porous film to restrain corrosion, which is similar to Cr6+ in dichromate sealing. The incorporation of Ce and Mo in the outer porous layer of the anodic film reduces the susceptibility to local corrosion. During the anodizing, there are many sites where metal precipitates are located. Mo and Ce concentrate at these sites to reduce the extent and activity of local cathodes. In Ce-Mo sealing, Ce and Mo produce a cooperative effect to improve the corrosion resistance of anodic films.
1) The Ce-Mo sealing makes the surface structure and morphology of anodic films uniform and compact. Ce and Mo exist throughout the anodic film.
2) In neutral NaCl solution, the high frequency impedance modulus of the anodic film sealed by Ce-Mo process is the largest in anodic films. Rp values increase and Cp values decrease. In acidic and basic NaCl solution, Ce-Mo sealing can also provide relatively high corrosion resistance.
References[1] CELATI N, CATHERINE M C S, KEDDAM M. Electrochemical impedance spectroscopy characterization of the protection by anodized layers on aluminium alloys [J]. Materials Science Forum, 1995, 192-194: 225-344.
[2] PATERMARAKIS G, PAPADREADIS N. Effect of the structure of porous anodic Al2O3 films on the mechanism of their hydrothermal treatment [J]. Electrochemica Acta, 1993, 38(10): 1413-1420.
[3] THOMPSON G E. Porous anodic alumina: fabrication, characterization and applications [J]. Thin Solid Films, 1997, 297: 192-201.
[4] SKELDON P, HABAZAKI H, THOMPSON G E, SHIMIZU K, WOOD G C. Formation of amorphous anodic oxide films of controlled composition on aluminium alloys [J]. Thin Solid Films, 1997, 300: 131-137.
[5] SUAY J J, GIMENEZ E. Characterization of anodized and sealed aluminium by EIS [J]. Corrosion Science, 2003, 45: 611-624.
[6] ZHU Z F. Technique status and development trends of surface treatment on aluminum alloys for architectural applications [J]. Electroplating & Finishing, 2005, 24(4): 14-17.
[7] HAO L, CHENG B R. Sealing processes of anodic coatings—past, present, and future [J]. Metal Finishing, 2000, 12(8): 8-18.
[8] ZHAO X H, ZUO Y, ZHAO J M. Electrochemical properties of anodized aluminum films in sodium chloride solution [J]. The Chinese Journal of Nonferrous Metals, 2004, 14(4): 562-567. (in Chinese)
[9] ZUO Y, ZHAO P H, ZHAO J M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solution [J]. Surface and Coatings Technology, 2003, 166: 237-242.
[10] HINTON B R W. Corrosion prevention and chromates, the end of an era? [J]. Metal Finishing, 1991, 89(9): 55-61.
[11] MANSFELD F, WANG Y. Development of “stainless” aluminum alloys by surface modification [J]. Materials Science and Engineering A, 1995, 198: 51-61.
[12] CHEN C, MANSFELD F. Corrosion protection of an Al6092/SiCp metal matrix composite [J]. Corrosion Science, 1997, 39(6): 1075-1082.
[13] HITZIG J, JUTTNER K, LORENZ W J. AC-impedance measurements on porous aluminium oxide films [J]. Corrosion Science, 1984, 24(11/12): 945-952.
[14] JUTTNER K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces [J]. Electrochimica Acta, 1990, 35(10): 1501-1508.
[15] MOUTARLIER V, GIGANDET M P, NORMAND B, PAGETTI J. EIS characterisation of anodic films formed on 2024 aluminium alloy, in sulphuric acid containing molybdate or permanganate species [J]. Corrosion Science, 2005, 47: 937-951.
[16] SNOGAN F, BLANC C, MANKOWSKI G, PEBERE N. Characterisation of sealed anodic films on 7050 T74 and 2214 T6 aluminium alloys [J]. Surface and Coatings Technology, 2002, 154: 94-103.
Foundation item: Project(50571006) supported by the National Natural Science Foundation of China
Corresponding author: ZUO Yu; Tel: 86-10-64434818; E-mail: zuoy@mail.buct.edu.cn
(Edited by YANG Bing)
Abstract: The elimination of toxic materials in sealing methods for anodic films on 1070 aluminum alloy was studied. The new process uses chemical treatments in cerium solution and an electrochemical treatment in a molybdate solution. Potentiodynamic polarization and electrochemical impedance spectroscopy(EIS) were used to study the influences of sealing methods on the corrosion behavior of anodic films in NaCl solutions. The results show that the Ce-Mo sealing makes the surface structure and morphology of anodic films uniform and compact. Ce and Mo produce a cooperative effect to improve the corrosion resistance of anodic films. Anodic films sealed by Ce-Mo provide high corrosion resistance both in acidic and basic solutions.


