Trans. Nonferrous Met. Soc. China 24(2014) 884-892
Morphological and crystallographic characteristics of lead powder obtained by electrodeposition from an environmentally friendly electrolyte
 D.
D.  1, Djendji Dj.
1, Djendji Dj.  2, Vesna M.
2, Vesna M.  3, Goran
3, Goran  4
4
1. ICTM- Institute of Electrochemistry, University of Belgrade,  12, Belgrade, Serbia;
12, Belgrade, Serbia;
2. Faculty of Sciences, University of Novi Sad, Trg D.  3, Novi Sad, Serbia;
3, Novi Sad, Serbia;
3. Department of material science, INN  , University of Belgrade, Belgrade, Serbia;
, University of Belgrade, Belgrade, Serbia;
4. Institute for Multidisciplinary Research, University of Belgrade, Kneza  1a, Belgrade, Serbia
1a, Belgrade, Serbia
Received 10 July 2013; accepted 16 September 2013
Abstract:
Lead powder obtained by potentiostatic electrodeposition from alkaline electrolyte, based on hydroxide ions, was investigated. The shape of lead crystals strongly depends on overpotentials of electrodeposition. The regular crystals are formed in the ohmic control. The shape of dendrites formed in the control of diffusion has a function of overpotentials of the electrodeposition. Increasing overpotential leads to branching of dendrites from primary type to those with developed tertiary branches. Formation of the very branchy dendrites of the strong (111) preferred orientation is explained on the basis of the affiliation of this electrolyte to the group of the complex Pb electrolytes.
Key words:
lead powder; electrodeposition; crystal; dendrite;
1 Introduction
In the form of powder, lead has been found multiple applications in many industries including oil and gas exploration, radiological medical protective clothing, industrial X-ray shield, golf club manufacture and anti-friction products [1]. In addition, powdered lead is used in lubricating grease to reduce or eliminate wear and as the basis for some corrosion-resistant paints.
The process of electrolysis is very suitable to get metals in the powder forms. Compared to other ways of powder synthesis, such as mechanic milling, chemical reaction and liquid metal atomization, the process of electrolysis requires lower capital investment and operational costs [2, 3]. Powder produced in this way is of high purity, can be easily pressed and sintered. The electrodeposition techniques are an environmentally friendly manner of powder production which can be done in a closed-circuit space [4].
Pb together with Ag, Cd, Zn, and Sn belong to the group of the normal metals [5, 6]. They are characterized by low melting points and high exchange current densities J0. Also, they show high overpotentials for hydrogen discharge. Electrodeposition of these metals belongs to fast electrochemical process, which enables the formation of metal powders at low overpotentials and current densities and hence with small spent energy.
Electrodeposited metal powders are mainly produced in a dendritic form [7]. Although dendrites have the most often shape of powder particles, some other forms, such as flakes, fibres, sponges, nanowires and cauliflower-like structures, can be also obtained by the process of electrodeposition [3,4,8-10]. The shape of powder particles depends on the nature of metals and electrodeposition conditions such as composition of the electrolyte, overpotential or current density applied and temperature [11-17]. For the process of lead electrodeposition, two types of electrolytes are used: acid (nitrate [18,19], iodide [20], bromide [20], acetate [21] and methanesulfonate [22]) and alkaline [23-26]. Unlike most of acid electrolytes which are toxic, alkaline electrolytes are more appropriate from the environmental standpoint [27]. Additionally alkaline solutions are less corrosive to plants and equipment.
The processes of lead electrodeposition from alkaline solutions, without or with the addition of the additives, are mainly analyzed by the application of cyclic voltammetry [23-26]. However, there are no enough data related to morphology of the lead deposits obtained from the alkaline electrolytes. The surface morphology is probably the most important property of electrodeposited metals which mainly depends on the kinetic parameters of the deposition process and the deposition overpotential or current density [7]. The aim of this study is to analyze electrodeposition of lead from the alkaline solution and to correlate the polarization characteristics of lead with morphology of lead deposits obtained by the potentiostatic electrodeposition. For the purpose, a solution containing 0.10 mol/L Pb(NO3)2 in 2.0 mol/L NaOH is analyzed because it gives the highest rate of deposition which is very suitable for industrial production [23].
2 Experimental
Electrodeposition of lead was performed in an open cell from 0.10 mol/L Pb(NO3)2+2.0 mol/L NaOH. Lead was electrodeposited potentiostatically at overpotentials of 20, 50, 80 and 100 mV with electricity of 0.50 mA·h/cm2.
Doubly distilled water and analytical grade chemicals were used for the preparation of the solutions for electrodeposition of lead. All electrodepositions were performed on vertical cylindrical copper electrodes. The geometric surface area of copper electrodes was 0.25 cm2. Reference and counter electrodes were pure lead. The counter electrode was lead foil with 0.80 dm2 surface area and was placed close to the cell walls. The reference electrode was wire of lead whose tips were positioned at a distance of about 0.2 cm from the surface of the working electrodes. The working electrodes were placed in the centre of cell, at the same location for each experiment. Electrodeposition of lead and polarization measurements were performed at a temperature of (22.0±0.5) °C.
In order to obtain a reproductive shape of the polarization curve for lead [19, 28], the following experimental procedure, usual for the recording of the polarization curves of fast electrochemical processes, was applied. The values of the current density obtained after stabilization of their values at every 5 mV were used for constructing the polarization curves. After the determined values of overpotential (the inflection point), the current increased dramatically and then, the values of the current density at the moment of reaching the selected values of the overpotential were used for the further recording of the polarization curve.
The morphologies of the obtained lead deposits were analyzed by scanning electron microscopy (SEM, TESCAN Digital Microscopy).
Powder particles obtained by tapping the lead deposits electrodeposited at overpotentials of 20, 50 and 100 mV were analyzed by the X-ray powder diffraction (XRD, Rigaku Ultima IV) with Cu Kα radiation, respectively.
3 Results and discussion
3.1 Polarization characteristics
Polarization curve for lead electrodeposition from 0.10 mol/L Pb(NO3)2 in 2.0 mol/L NaOH is shown in Fig. 1. The beginning of the lead electrodeposition process is shifted to the higher overpotential by about 5 mV. The characteristic of a part of polarization curve denoted Part (I) is the linear dependence of the current density on the overpotential. In this range of overpotentials, the electrodeposition process is under ohmic control. The limit of the ohmic control is at an overpotential of 25 mV. The ohmic control is followed by the diffusion control in the range of overpotentials between 25 and 80 mV (Part (II) at the polarization curve). The beginning of the plateau of the limiting diffusion current density is determined as intersect of straight lines joining current densities in the ohmic and diffusion control of electrodeposition [21], as shown in the inset of Fig. 1. The end of the plateau of the limiting diffusion current density corresponds to the inflection point at the polarization curve (φ=80 mV). Then, the plateau of the limiting diffusion current density determined in this way is between 39.5 and 80 mV. After the inflection point, the current density increases quickly with further increasing of the overpotential. The fast increase of current density corresponds to the third part of the polarization curve (Part (III)).
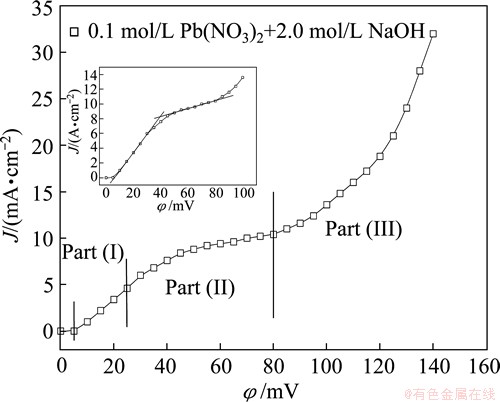
Fig. 1 Polarization curve for lead electrodeposition from 0.10 mol/L Pb(NO3)2+2.0 mol/L NaOH
3.2 Morphological and crystallographic analysis of Pb powdered deposits
Morphologies of lead deposits obtained by electrodeposition in the potentiostatic mode at overpotentials corresponding to different positions at the polarization curve are shown in Figs. 2-5.

Fig. 2 Regular crystals of lead obtained by electrodeposition at overpotential of 20 mV (ohmic control of electrodeposition)
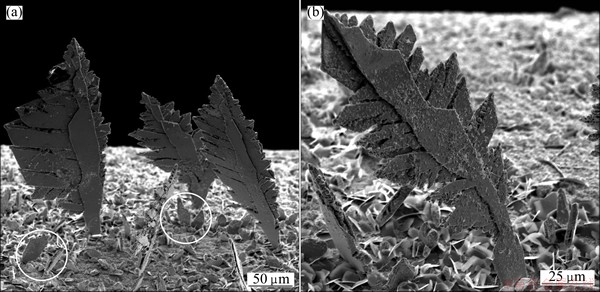
Fig. 3 Typical fern-like dendrites obtained by electrodeposition at overpotential of 50 mV (plateau of limiting diffusion current density)
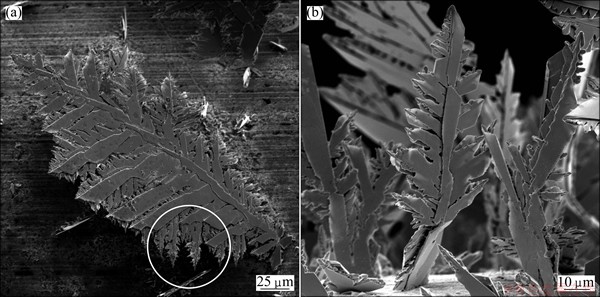
Fig. 4 Fern-like dendrites obtained by electrodeposition at overpotential of 80 mV (end of plateau of limiting diffusion current density)

Fig. 5 Fern-like dendrites obtained by electrodeposition at overpotential of 100 mV (zone of fast increase of current density with increasing overpotential)
Figure 2 shows the regular crystals obtained in the ohmic control at an overpotential of 20 mV. As expected, the fern-like dendrites were dominant morphological forms obtained during the diffusion controlled electrodeposition. The typical dendrites obtained at an overpotential of 50 mV are shown in Fig. 3. Aside from the dendrites, the small irregular crystals (precursors of dendrites) were also formed by the electrodeposition at 50 mV (in the circles in Fig. 3(a)). The more branchy dendrites were formed during electrodeposition at an overpotential of 80 mV which corresponds to the end of the plateau of the limiting diffusion current density (Fig. 4). It is necessary to note that the dendrites have only morphological forms formed during the electrodeposition at this overpotential. The very branchy dendrites, as the only type of the surface morphology, were also obtained at 100 mV (Fig. 5) in the zone of the fast growth of the current density with the overpotential.
According to results of WRANGLEN [29], a dendrite consists of a stalk and branches (primary, secondary and tertiary), which resemble a tree. The flat and fern-like dendrites are referred as two-dimensional (2D) ones. The primary (P) type of dendrite consists of the stalk and primary branches. If the primary branches develop secondary branches in turn, the dendrite is called secondary (S). The tertiary branches are developed from the secondary ones. From Fig. 3, it can be clearly seen that the primary (P) type of a dendrite is formed at 50 mV. Increasing overpotential causes branching of dendrites, and the mixture of primary (P) and secondary (S) dendrites is predominately formed at 80 mV (Fig. 4). Finally, the dendrites of S type are predominately formed at 100 mV (Fig. 5). From Fig. 4(a), it can be observed that the tertiary branches developed from the secondary ones which additionally increase a ramification of the S dendrites. The two-dimensional (2D) shape of the fern-like dendrites is clearly visible in Figs. 3-5. These fern-like dendrites are denoted as 2D [110] 60o with an angle of 60° between the stalk and the branches [29].
Electrodeposition of lead from 0.10 mol/L Pb(NO3)2 +2.0 mol/L NaOH occurs, similar to Pb electrodeposition from acid electrolytes [19,21], under the conditions of the mixed ohmic-diffusion control. In relation to acid nitrate and acetate electrolytes, the beginning of the lead electrodeposition from this solution is shifted to the higher overpotential by about 5 mV, indicating a sensitivity of the reaction of Pb electrodeposition to the type of electrolyte. Also, Pb electrodeposition reaction is sensitive to the substrate type. For example, Pb electrodeposition on SS316 stainless steel occurs at a slightly higher overpotential than that at a platinum one [24]. The mixed ohmic-diffusion or even full ohmic control [11,18,30] is one of main characteristics of the fast electrochemical processes characterized by J0→∞ (“normal metals” [5,6]) such as the processes of silver electrodeposition from the basic electrolytes [31-34]. The ohmic control is followed by formation of characteristic morphological forms. In the case of lead, the regular crystals shown in Fig. 2 are obtained in the ohmic control. The shape of regular crystals does not depend on the type of electrolyte [21].
The dendritic growth is initiated at some overpotential which belongs to the diffusion part at the polarization curve. This overpotential represents the critical overpotential for dendritic growth initiation, φi, and it is overpotential at which the system is in the diffusion control. The inflection point at the polarization curve (the end of the plateau of the limiting diffusion current density) corresponds to the critical overpotential for dendritic growth instantaneous, φc, and it is overpotential at which the diffusion control becomes complete. The proof for this assertion is formation of only dendrites at an overpotential of 80 mV, corresponding to the inflection point (the end of the plateau of the limiting diffusion current density, as shown in Fig. 4). Contrary, the mixture of irregular crystals and dendrites is formed at 50 mV corresponding to the diffusion part at the polarization curve (25-80 mV, as shown in Fig. 3). After the inflection point, the system remains diffusion controlling and the fast increase of the current density with increasing the overpotential can be ascribed to instantaneous formation and growth of dendrites and to the strong increase of the surface area.
From the electrochemical point of view, a dendrite is defined as an electrode surface protrusion that grows under activation control, while electrodeposition to the macroelectrode is predominantly under diffusion control [7,34-36]. Using this definition of dendrite, the sudden and rapid increase of the current density with increasing the overpotential after the inflection point (Part (III) in the polarization curve) can be mainly ascribed to the activation controlled electrodeposition at the tips of the formed dendrites [18,19,21,34], where the tips of all three types of branches (primary, secondary and tertiary ones) contribute to the overall control of electrodeposition process. During this dendritic growth, the outer limit of the diffusion layer of the macroelectrode is disrupted.
The crystallographic characteristics of Pb crystals obtained by tapping the lead deposits electrodeposited at overpotentials of 20 (the ohmic control), 50 (the diffusion control) and 100 mV (the zone of the fast increase of the current density with the overpotential) were examined by the X-ray diffraction (XRD) analysis. The XRD patterns of the powder particles obtained in such way are shown in Fig. 6. In all powder particles, lead crystallites are predominately oriented in the (111) plane. The presence of Pb crystallites oriented in the (200), (220), (311) and (331) planes is negligible in the powder particles obtained at an overpotential of 20 mV. Increasing overpotential leads to the appearance of Pb crystallites oriented in the (200), (220), (311) and (331) planes, and it can be noticed that the ratio of crystallites oriented in these planes is considerably smaller than that oriented in the (111) plane. The ratio of crystallites oriented in the (200), (220), (311) or (331) planes increases with increasing the overpotential of electrodeposition. For the powder particles obtained at 100 mV, the size of crystallites is determined by Williamson-Hall method and is estimated to be 36.5 nm. The quantitative analysis of this Pb powder shows 100% purity, indicating the significance of the electrolysis processes in the production of powdered forms of high purity. The strong (111) preferred orientation can be discussed as follows: for face centered cubic structure like Pb, the surface energy of (111) plane is lower than that of other planes, since the surface energies follow the trend g111<>100<>110 [28,37]. For the same reason, at constant overpotential, the rates of electrodeposition increase in the order of (110)>(100)>(111) [38]. The different deposition rates onto different crystal faces enable the classification of these crystal planes in the two groups [28]. The first group is situated (111) plane and this plane is denoted as a slow-growing one. The (200), (220), (311) and (331) planes are fast-growing ones and belong to the second group [28]. In the growth process, the slow-growing (111) plane will survive [38], causing the predominant orientation of Pb crystallites in this plane. Simultaneously, the other planes (fast-growing ones) disappear, which explain the considerably smaller orientation of Pb crystallites in the (200), (220), (311) and (331) planes than those in the (111) plane. The growth centres present in the interior of the crystal faces (centre type of growth centres) are responsible for the orientation of Pb crystallites in the (111) plane [30]. The crystal growth based on these growth centres occurs at low current densities and overpotentials [29,30]. The origin of Pb crystallites oriented in the (200), (220), (311) and (331) planes is of the growth centres present on the edges and corners (edge and corner type of growth centres), and the growth of crystals based on these growth centres occurs at high current densities and overpotentials.
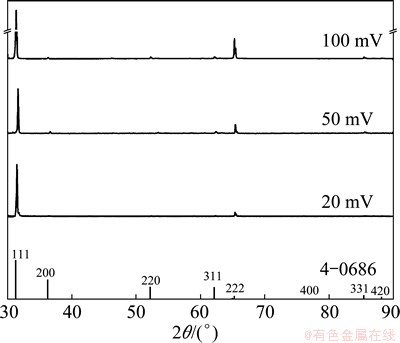
Fig. 6 XRD patterns of powder particles obtained by tapping lead deposits electrodeposited at overpotentials of 20, 50 and 100 mV
3.3 Correlation between morphological and crystallographic characteristics of Pb powder particles
The morphological and crystallographic aspects of formation of powder particles can not be analyzed separately by electrochemical ones. Schematic diagram illustrating the overpotential dependent formation and growth of fern-like dendrites is shown in Fig. 7. At the low overpotential (Fig. 7(a)), current lines are equally distributed and regular crystals like those shown in Fig. 2 are formed. Crystal growth based only on the growth centres present in the interior of the crystal faces is responsible for formation of the regular crystals, which is confirmed by the X-ray diffraction analysis of this surface morphology type. Namely, the X-ray diffraction analysis (Fig. 6) shows that they represent single crystals of the (111) orientation. Increasing overpotential leads to a concentration of current lines in the first place at the tips and corners of growing forms causing faster growth on them than at the sides of crystals and in the vicinity of the electrode surface (the current density distribution effect) [39]. Hence, the current densities are higher at the tips and corners of the growing forms (the growth based on edge and corner growth centres) than those at their sides (the growth based on growth centres of the centre type). As a result of this, irregular crystals or precursors of dendrites are formed (Fig. 7(b)). The origin of Pb crystallites oriented in the (200), (220), (311) and (331) planes is just of crystal growth on the tips and corners of growing crystals. Simultaneously, the sides of the crystals are constructed from Pb crystallites oriented in the (111) plane [30]. The typical irregular crystals (precursors of dendrites) are shown in the circle in Fig. 3(a). Formation of the irregular crystals is one of the main characteristic of electrodeposition process at the beginning of the diffusion control of the electrodeposition before the plateau of the limiting diffusion current density is reached. Also, irregular crystals are formed at the beginning of the zone of the fast increase of the current density with the overpotential in the conditions of the full ohmic control of electrodeposition [18,30]. The current density, and hence, concentration of current lines at the growing crystals increase with increasing of the overpotential, leading to the appearance of dendrites at the higher overpotentials (Fig. 7(c)). For reasons of simplification, the only one part of current lines is shown in Fig. 7. During the growth of dendrites the current densities increase exponentially. The larger the applied overpotential is, the faster the exponential increase of the current density during electrodeposition is [18]. Due to the increase of the number of growth centres at which a concentration of current lines takes place, branching of dendrites is observed resulting in the development of primary, secondary and tertiary branches in dendrites (Figs. 7(d) and (e) and Figs. 3-5).
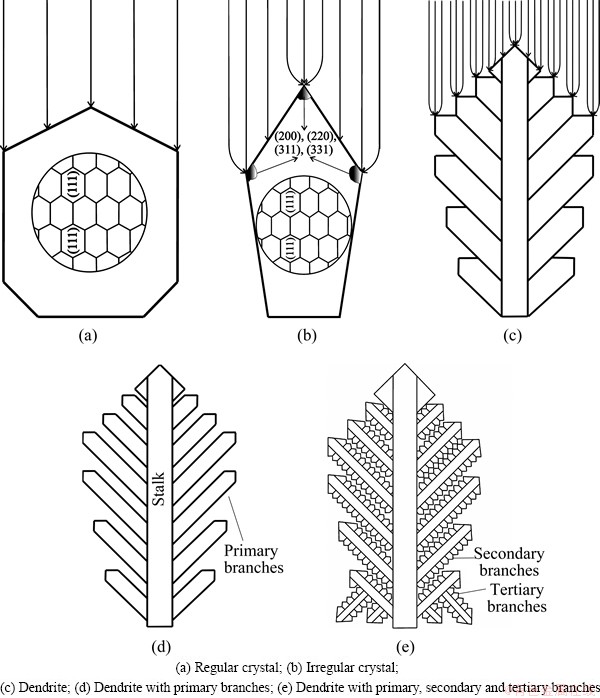
Fig. 7 Schematic diagram illustrating overpotential dependent growth of fern-like dendrites
For this alkaline electrolyte, the both ohmic and diffusion parts of the polarization curve are shifted to higher overpotentials in relation to the acid electrolytes based on nitrate and acetate ions (for the same concentration of Pb2+ ions). For the nitrate and acetate electrolytes, the ranges of the ohmic control are: 0-15 mV (nitrate electrolyte) and 0-20 mV (acetate one) [21]. Simultaneously, the intervals of overpotentials corresponding to the diffusion control are: 15-55 mV (the plateau of the limiting diffusion current density: 28.5-55 mV) for the nitrate and 20-70 mV (the plateau of the limiting diffusion current density: 33-70 mV) for the acetate electrolytes [21]. The increasing trend of these values in row nitrate<> Lead dendrites formed from the alkaline electrolyte (Figs. 3-5) are more similar to those obtained from the acetate than those formed from the nitrate electrolytes. The ramified dendrites belonging to the secondary (S) type are formed from both acetate and alkaline electrolytes while the needle-like and primary (P) dendrites are formed from the nitrate electrolyte [18,19,21,28,30]. The common characteristic of the acetate and alkaline electrolytes is their affiliation to the group of complex electrolytes. Namely, lead makes different complexes with hydroxide ions, such as [PbOH]+, [Pb(OH)2], [Pb(OH)3]-, [Pb2(OH)]3+, [Pb3(OH)4]2+, [Pb4(OH)4]4+ and [Pb6(OH)8]4+ [40,41]. In the very alkaline electrolyte solution, pH over 12 (as in this case), [Pb(OH)3]- complex becomes a major species in the solution [42-44], and probably the reduction of lead occurs entirely through this complex and not through Pb2+ ions [23]. The main differences between the complex and basic electrolytes are larger in the plateaus of the limiting diffusion current density for the complex than for the basic electrolytes. Simultaneously, dendrites obtained by electrodeposition from the complex electrolytes are branchier than those electrodeposited from the basic electrolytes. The process of complex formation lowers the exchange current density, J0, and decreases the rate of electrochemical process. Due to the lack of a precise and reference method for the determination of J0 for the majority of normal metals, such as Pb, Ag, Sn and Zn, the analysis of the polarization characteristics and the surface morphology of dendrites formed in the diffusion control can be very good auxiliary diagnostic criteria for the comparison of electrodeposition systems characterized by the large exchange current density (J0→∞) with point of view of the different J0 values (i.e. rate of electrochemical process). The degree of the change of the both polarization and morphological characteristics can be correlated with the stability of formed complexes. Namely, Pb (II) ions form relatively weak complexes with acetate ions. The stability constant for the acetate complexes are lg K1= 2.33 and lg β2=3.60. On the other hand, Pb makes stronger complex with hydroxide ions than with the acetate ions. For example, for [Pb(OH)3]- complex, which is primary species in very alkaline solutions (2.0 mol/L NaOH), lg β3=13.3 [40,44]. This constant is about 5×109 times the constant for the acetate complex. Anyway, when the stronger complex is formed, the stronger effect on the polarization characteristics and surface morphology of electrodeposited metal is observed. In this case, this is manifested through the increase of the plateau of the limiting diffusion current density and formation of larger number of dendrites of S type, as well as by the appearance of tertiary branches in the dendrites of S type from the alkaline electrolyte in relation to the acetate one. 4 Conclusions 1) Electrodeposition of lead occurred, similar to electrodeposition of lead from acid (nitrate and acetate electrolytes), in the mixed ohmic-diffusion control. The regular crystals were formed in the ohmic control. The shape of these crystals did not depend on the type of electrolyte (Part (I) at the polarization curve). The irregular crystals and dendrites of the different shapes were formed in the diffusion control (Part (II) at the polarization curve). Very branchy dendrites were formed in the zone of the fast increase of the current density with increasing the overpotential (Part (III) at the polarization curve). 2) The shape of dendrites strongly depended on overpotential of the electrodeposition. The branching of dendrites was observed with increasing the overpotential. The dendrites composed of a stalk and three types of branches were formed: primary, secondary and tertiary ones. The observed dendrites were more similar to those obtained from the acetate electrolyte than those obtained from the nitrate one. Formation of very branchy dendrites was discussed on the basis of the fact that this alkaline electrolyte belongs to the group of the complex electrolytes. References [1] Nuclead Co. Inc. and Sharp Manufacturing Inc. Lead powder applications [P/OL]. 2014. http://www.nuclead.com/leadpowderapps. html. [2] GERMAN R M. Powder metallurgy science [M]. 2nd ed. Princeton: Metal Powder Industries Federation, 1994. [3] [4] ORHAN G, HAPCI G. Effect of electrolysis parameters on the morphologies of copper powder obtained in a rotating cylinder electrode cell [J]. Powder Technol, 2010, 201: 57-63. [5] WINAND R. Electrodeposition of metals and alloys—New results and perspectives [J]. Electrochim Acta, 1994, 39: 1091-1105. [6] KOZLOV V M, PERALDO BICELLI L. Influence of the nature of metals on the formation of the deposit`s polycrystalline structure during electrocrystallization [J]. J Cryst Growth, 1999, 203: 255-260. [7] POPOV K I, [8] POPOV K I, [9] NI Y, ZHANG Y, ZHANG L, HONG J. Mass synthesis of dendritic Bi nanostructures by a facile electrodeposition route and influencing factors [J]. Cryst Eng Comm, 2011, 13: 794-799. [10] YANG M. Fern-shaped bismuth dendrites electrodeposited at hydrogen evolution potentials [J]. J Mater Chem, 2011, 21: 3119-3124. [11] [12] [13] [14] HAN J, LIU J. Electrodeposition of crystalline dendritic silver in 12-tungstosilicate acid system [J]. J Nanoeng Nanomanuf, 2012, 2: 171-174. [15] MANDKE M V, HAN S H, PATHAN H M. Growth of silver dendritic nanostructures via electrochemical route [J]. CrystEngComm, 2012, 14: 86-89. [16] WANG J, WEI L, ZHANG L, ZHANG Y, JIANG C. Electrolytic approach towards the controllable synthesis of symmetric, hierarchical, and highly ordered nickel dendritic crystals [J]. CrystEngComm, 2012, 14: 1629-1636. [17] SIVASUBRAMANIAN R, SANGARANARAYANAN M V. Electrodeposition of silver nanostructures: From polygons to dendrites [J]. Cryst Eng Comm, 2013, 15: 2052-2056. [18] [19] [20] MOSTANY J, PARRA J, SCHARIFKER B R. The nucleation of lead from halide-containing solutions [J]. J Appl Electrochem, 1986, 16: 333-338. [21] [22] PLETCHER D, WILLS R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead(II). Part II. Flow cell studies [J]. Phys Chem Chem Phys, 2004, 6: 1779-1785. [23] CARLOS I A, MALAQUIAS M A, OIZUMI M M, MATSUO T T. Study of the influence of glycerol on the cathodic process of lead electrodeposition and on its morphology [J]. J Power Sources, 2001, 92: 56-64. [24] WONG S M, ABRANTES L M. Lead electrodeposition from very alkaline media [J]. Electrochim Acta, 2005, 51: 619-626. [25] CARLOS I A, SIQUEIRA J L P, FINAZZI G A, de ALMEIDA M R H. Voltammetric study of lead electrodeposition in the presence of sorbitol and morphological characterization [J]. J Power Sources, 2003, 117: 179-186. [26] GU Ying-ying, ZHOU Qiong-hua, YANG Tian-zu, LIU Wei, ZHANG Du-chao. Lead electrodeposition from alkaline solutions containing xylitol [J].Transactions of Nonferrous Metals Society of China,2011, 21: 1407-1413. [27] JORDAN M. Electrodeposition of lead and lead alloys [M]// SCHLESINGER M, PAUNOVIC M. Modern electroplating. John Wiley & Sons, 2010: 251. [28] [29] WRANGLEN G. Dendrites and growth layers in the electrocrystallization of metals [J]. Electrochim Acta, 1960, 2: 130-146. [30] [31] POPOV K I, [32] POPOV K I, [33] POPOV K I, [34] POPOV K I, [35] [36] DIGGLE J W, [37] XIA Y, XIONG Y, LIM B, SKRABALAK S E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? [J]. Angew Chem Int Ed, 2009, 48: 60-103. [38] BOCKRIS J O` M, REDDY A K N, GAMBOA-ALDECO M. Modern electrochemistry 2A, fundamentals of electrodics [M]. New York: Kluwer Academic/Plenum Publishers, 2000: 1333. [39] POPOV K I, [40] SMITH R M, MARTELL A E. NIST standard reference database 46, nist critically selected stability constants of metal complexes database, version 3.0 [M]. Gaithersburg, MD 20899, USA. Department of Commerce, National Institute of Standards and Technology. [41] KRATGEN J. Atlas of metal-ligand equilibria in aqueous solution [M]//In Analitical Chemistry. Chichester: Ellis Horwood, 1978. [42] CUKROWSKA E, CUKROWSKI I. Protonation constant of monoaza-12-crown-4 ether and stability constants with selected metal ions in aqueous solution in the presence of an excess of sodium ion: A potentiometric and differential pulse polarographic study at fixed ligand to metal ratio and varied pH [J]. Talanta, 1998, 47: 1175-1189. [43] PEREIRA M, MANTAS P Q. Preparation of oxalate precursor of PLZT. Characterization of the individual components [J]. J Eur Ceram Soc, 1998, 18: 565-582. [44] WANG Yun-yan, CHAI Li-yuan, CHANG H, PENG Xiao-yu, SHU Yu-de. Equilibrium of hydroxyl complex ions in Pb2+-H2O system [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 458-462. 1. ICTM- Institute of Electrochemistry, University of Belgrade, 2. Faculty of Sciences, University of Novi Sad, Trg D. 3. Department of material science, INN 4. Institute for Multidisciplinary Research, University of Belgrade, Kneza 摘 要:采用扫描电镜(SEM)研究在碱性电解液中通过恒电位沉积得到的铅粉。结果表明:铅晶体的形状主要取决于电沉积的过电位。通过欧姆控制步骤可以得到规则晶体。通过扩散控制得到的枝晶形状和电沉积的过电位有关系。不断增加的过电位导致枝晶的分枝从原始类型变成发达的三级分枝。基于该电解液属于络合铅电解液,解释具有强烈的(111)择优取向的枝晶分枝的形成原因。 关键词:铅粉;电沉积;晶体;枝晶 (Edited by Chao WANG) Foundation item: Project (172046) supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia Corresponding author: DOI: 10.1016/S1003-6326(14)63139-3 M G, POPOV K I. Electrochem [M/OL]. Encyclopedia, 2005, http://electrochem.cwru.edu/ed/encycl/.
M G, POPOV K I. Electrochem [M/OL]. Encyclopedia, 2005, http://electrochem.cwru.edu/ed/encycl/. S S, GRGUR B N. Fundamental aspects of electrometallurgy [M]. New York: Kluwer Academic/Plenum Publishers, 2002: 78-89.
S S, GRGUR B N. Fundamental aspects of electrometallurgy [M]. New York: Kluwer Academic/Plenum Publishers, 2002: 78-89. M I. The mechanism of formation of coarse and disperse electrodeposits [C]//WHITE R E, CONWAY B E, BOCKRIS J. O ' M. Modern Aspects of Electrochemistry. New York: Plenum Press, 1996: 261-312.
M I. The mechanism of formation of coarse and disperse electrodeposits [C]//WHITE R E, CONWAY B E, BOCKRIS J. O ' M. Modern Aspects of Electrochemistry. New York: Plenum Press, 1996: 261-312. V D,
V D,  N D,
N D, 
 B M, POPOV K I. Morphology of different electrodeposited pure metal powders [C]//
B M, POPOV K I. Morphology of different electrodeposited pure metal powders [C]//  S S. Electrochemical Production of Metal Powders. Springer, 2012: 63-123.
S S. Electrochemical Production of Metal Powders. Springer, 2012: 63-123. N D, POPOV K I. Electrodeposition of copper powders and their properties [C]//
N D, POPOV K I. Electrodeposition of copper powders and their properties [C]//  S S. Electrochemical Production of Metal Powders. Springer, 2012: 125-185.
S S. Electrochemical Production of Metal Powders. Springer, 2012: 125-185. S S,
S S,  N D,
N D,  P M, POPOV K I,
P M, POPOV K I,  N S. Electroless deposition and electrodeposition of metallic powders: A comparison [J]. ECS Trans, 2011, 33: 7-31.
N S. Electroless deposition and electrodeposition of metallic powders: A comparison [J]. ECS Trans, 2011, 33: 7-31. N D,
N D,  G,
G,  U. Formation of two-dimensional (2D) lead dendrites by application of different regimes of electrolysis [J]. J Solid State Electrochem, 2012, 16: 2121-2126.
U. Formation of two-dimensional (2D) lead dendrites by application of different regimes of electrolysis [J]. J Solid State Electrochem, 2012, 16: 2121-2126. N D, POPOV K I,
N D, POPOV K I,  P M,
P M,  G. A new insight into the mechanism of lead electrodeposition: Ohmic-diffusion control of the electrodeposition process [J]. J Electroanal Chem, 2013, 691: 66-76.
G. A new insight into the mechanism of lead electrodeposition: Ohmic-diffusion control of the electrodeposition process [J]. J Electroanal Chem, 2013, 691: 66-76. N D,
N D,  Dj Dj,
Dj Dj,  P M,
P M,  B,
B,  G. Influence of the complex formation on the morphology of lead powder particles produced by the electrodeposition processes [J]. Adv Powder Technol, 2013, 24: 674-682.
G. Influence of the complex formation on the morphology of lead powder particles produced by the electrodeposition processes [J]. Adv Powder Technol, 2013, 24: 674-682. N D,
N D,  V M,
V M,  G,
G,  P M,
P M,  M G. Influence of the type of electrolyte on morphological and crystallographic characteristics of lead powder particles [J]. J Serb Chem Soc, 2013, 78: 1387-1395.
M G. Influence of the type of electrolyte on morphological and crystallographic characteristics of lead powder particles [J]. J Serb Chem Soc, 2013, 78: 1387-1395. N D,
N D,  V M,
V M,  G. Morphological and crystallographic characteristics of electrodeposited lead from the concentrated electrolyte [J]. RSC Adv, 2013, 3: 7466-7471.
G. Morphological and crystallographic characteristics of electrodeposited lead from the concentrated electrolyte [J]. RSC Adv, 2013, 3: 7466-7471. P M, GRGUR B N. Physical and mathematical models of an inert macroelectrode modified with active hemispherical microelectrodes [J]. Electrochim Acta, 2007, 52: 4696-4707.
P M, GRGUR B N. Physical and mathematical models of an inert macroelectrode modified with active hemispherical microelectrodes [J]. Electrochim Acta, 2007, 52: 4696-4707. P M,
P M,  N D. The effect of morphology of activated electrodes on their electrochemical activity [C]//
N D. The effect of morphology of activated electrodes on their electrochemical activity [C]//  S S. Electrodeposition: Theory and Practice. Springer, 2010: 163-213.
S S. Electrodeposition: Theory and Practice. Springer, 2010: 163-213. P M,
P M,  S B,
S B,  N D. Polarization curves in the Ohmic controlled electrodeposition of metals [J]. Electrochim Acta, 2009, 54: 2924-2931.
N D. Polarization curves in the Ohmic controlled electrodeposition of metals [J]. Electrochim Acta, 2009, 54: 2924-2931. N D. General theory of disperse metal electrodeposits formation [C]//
N D. General theory of disperse metal electrodeposits formation [C]//  S S. Electrochemical Production of Metal Powders. Springer, 2012: 1-62.
S S. Electrochemical Production of Metal Powders. Springer, 2012: 1-62. A R, POPOV K I. Transport controlled deposition and dissolution of metals [C]//CONWAY B E, BOCKRIS J O` M. Modern Aspects of Electrochemistry. New York: Plenum Press, 1972: 199-313.
A R, POPOV K I. Transport controlled deposition and dissolution of metals [C]//CONWAY B E, BOCKRIS J O` M. Modern Aspects of Electrochemistry. New York: Plenum Press, 1972: 199-313. A R, BOCKRIS J O ' M. The mechanism of the dendritic electrocrystallization of zinc [J]. J Electrochem Soc, 1969, 116: 1503-1514.
A R, BOCKRIS J O ' M. The mechanism of the dendritic electrocrystallization of zinc [J]. J Electrochem Soc, 1969, 116: 1503-1514. P M,
P M,  N D. A mathematical model of the current density distribution in electrochemical cells [J]. J Serb Chem Soc, 2011, 76: 805-822.
N D. A mathematical model of the current density distribution in electrochemical cells [J]. J Serb Chem Soc, 2011, 76: 805-822.在环境友好的电解液中电沉积铅粉末的形态和晶体学特征
 D.
D.  1, Djendji Dj.
1, Djendji Dj.  2, Vesna M.
2, Vesna M.  3, Goran
3, Goran  4
4 12, Belgrade, Serbia;
12, Belgrade, Serbia; 3, Novi Sad, Serbia;
3, Novi Sad, Serbia; , University of Belgrade, Belgrade, Serbia;
, University of Belgrade, Belgrade, Serbia; 1a, Belgrade, Serbia
1a, Belgrade, Serbia D.
D.  ; Tel/Fax: +381-11-3370389; E-mail: nnikolic@tmf.bg.ac.rs
; Tel/Fax: +381-11-3370389; E-mail: nnikolic@tmf.bg.ac.rs
Abstract: Lead powder obtained by potentiostatic electrodeposition from alkaline electrolyte, based on hydroxide ions, was investigated. The shape of lead crystals strongly depends on overpotentials of electrodeposition. The regular crystals are formed in the ohmic control. The shape of dendrites formed in the control of diffusion has a function of overpotentials of the electrodeposition. Increasing overpotential leads to branching of dendrites from primary type to those with developed tertiary branches. Formation of the very branchy dendrites of the strong (111) preferred orientation is explained on the basis of the affiliation of this electrolyte to the group of the complex Pb electrolytes.


