Trans. Nonferrous Met. Soc. China 26(2016) 339-347
Ductility and hardness of chloride cleaned AA6011/SiCp composites
S. O. ADEOSUN1, E. I. AKPAN2, O. P. GBENEBOR1, S. A. BALOGUN3
1. Department of Metallurgical and Materials Engineering, University of Lagos, Lagos 100213, Nigeria;
2. Department of Materials and Production Engineering, Ambrose Alli University, Ekpoma 310101, Nigeria;
3. Department of Mechanical and Biomedical Engineering, Bells University of Technology, Ota 110001, Nigeria
Received 23 March 2015; accepted 14 October 2015
Abstract:
The ductility and hardness of AA6011/SiCp composites using NaCl, SnCl2, NH4Cl and PdCl2 as wetting reagents were investigated. SiCp was cleaned with the wetting reagents, and used as reinforcement in AA6011 alloy using the stir casting method. Ductility and hardness responses of the composites were measured using standard methods. Microstructural features were examined using scanning electron microscopy and the phases were determined with the help of an X-ray diffractometer. The results show that for all wetting agents, the increase in cleaning time leads to initial increase in ductility to a certain value, but a decrease afterwards with further increase in cleaning time. The best combination of hardness (BHN 57.88) and ductility (11.91%) was shown under conditions of 40 g/L SnCl2 and cleaning time of 60 min. A minor formation of Al4C3 was noted in diffraction patterns, indicating that the formation of deleterious precipitate was hindered by the cleaning process.
Key words:
aluminium alloy composite; silicon carbide; wetting agent; ductility; hardness; reinforcement;
1 Introduction
Recent studies on the production of metal matrix composites with improved properties for automobile and aerospace applications have been focused on the use of ceramic particles as reinforcements in aluminium. This is because these composites offer improvements in wear resistance, strength, structural efficiency and reliability. Aluminium has been reinforced with ceramic particles such as Al2O3, ZrO2, SiCp, iron fillings, electric arc furnace dust, graphite and mica [1-9]. Among these, Al/SiCp composites are the most researched for structural and aerospace applications because of their high modulus, high specific stiffness, low thermal expansion coefficient, good workability and excellent wear resistance. However, these composites are found to possess low ductility [10,11].
A review of studies on the ductility of metal matrix composites indicates that the ductility is dependent on factors such as fabrication method, particle adhesion to matrix and particle de-cohesion. ZAKERIA and RUDI [12] compared hot extrusion and hot pressing of metal matrix composites and reported the improvement in ductility of hot-extruded composites. DENG and CHAWLA [13] and TZAMTZIS et al [14] attributed low ductility of the composites to the agglomeration of particles in the ductile matrix caused by the production method. Another study by LING et al [15] using sintering and isostatic pressing method shows the improvement in ductility of composites with isostatic pressing compared with other fabrication methods. The work of HONG and KAO [16] also demonstrates that resistance sintering of mechanically alloyed powders leads to reasonable improvement in ductility. These findings may stand true because shaping processes are known to influence the microstructure of materials and consequently the mechanical properties. However, some other studies have shown that the three most significant contributions to mechanical properties of the AA6011/SiCp composites are particle clustering, formation of brittle phases of Al4C3 produced due to undesirable reaction between SiCp and molten aluminium and poor interfacial adhesion between the reinforcement and matrix [13,14,17-19].
The presence of brittle Al4C3 particles is detrimental to ductility because they act as de-cohesion nucleation site bringing about early fracture [14]. On the other hand, poor interfacial adhesion is detrimental to ductility due to de-bonding of reinforced particles during straining [20,21]. TZAMTZIS et al [14] also attributed the problem of particle clustering to chemical bonding of the particle and the matrix. In the previous study [3], we reported minimum formation of the Al4C3 intermetallic due to mould wall preheating and this promotes steady increase in ductility of the composites. On the other hand, SOBCZAK et al [22], LAURENT et al [23], ALONSO et al [24] and CAROTENUTO et al [25] have shown that reactive wetting agents improve wettability and eliminate the formation of Al4C3. It is therefore believed that wetting agents of this category will lead to the improvement in ductility and related properties.
Our preliminary study [19] showed that SnCl2-cleaned SiCp led to the improvement in ductility of the composites with the increase in cleaning concentration and time up to 120 min compared with untreated SiCp. In this study, the effects of SnCl2, NaCl, PdCl2 and NH4Cl as wetting agents on the ductility and hardness of AA6011/SiCp composite produced by stir casting were investigated. Casting schedules were designed to test the effect of cleaning time and concentration of the wetting agent on the ductility and hardness of the composites.
2 Experimental
2.1 Cleaning of SiCp
Cleaning of SiCp was done by placing 10 g of SiCp in a plastic cup and the known concentration of the prepared reagent was poured into the cup well above the level of the particles and stirred periodically within the specified time. A filter paper was placed over a cup and the solution was poured into it to filter the cleaned SiCp. Wetted SiCp was then allowed to dry still in air before casting. Cleaning time used in the study ranged between 60 and 160 min at an interval of 2 min.
2.2 Casting of Al/SiC composites
AA6011 ingot was charged into a crucible in a muffle furnace and heated to a temperature of about 800 °C for about 1 h to obtain molten aluminium alloy. The molten aluminium alloy was then transferred to a steel cup and 10 g of wetted SiCp was added and stirred properly with the aid of a glass rod. The mixture was poured into the mould and allowed to solidify. Cast samples were machined to standard size for tensile, hardness and metallographic specimens.
2.3 XRD pattern
An X’Pert Pro MPD model diffractometer was used to observe the diffraction pattern of the composite. The diffraction patterns were collected by mounting composite in a specially designed sample holder (0.1 g of powder composite was placed on a rectangular flat glass (45 cm × 3.6 cm) to about 1 mm in thickness to cover a rectangular space (1.5 cm × 2 cm) using 2θ range of 5°-99° in step size of 0.06685°, using a NO filter). The diffraction patterns were collected within 7-8 min with the X-ray beam set to 40 kV.
2.4 SEM image
An ASPEX 3020 model variable pressure scanning electron microscope (SEM) was used to observe the morphological features of all samples. Samples to be observed were mounted on conductive carbon imprint left by the adhesive tape prepared by placing the samples on the circular holder and coated for 5 min to enable it to conduct electricity using an E-1010 Hitachi model machine. The SEM structural analysis was carried out at Materials Science Laboratory of Kwara State University, Malete (KWASU), Nigeria.
2.5 Tensile testing
Tensile test was conducted at Federal Institute of Industrial Research Oshodi (FIIRO), Lagos State, Nigeria, using a digital Instron universal tensile testing machine (M500). Each specimen was placed in the testing machine, locked at each end, and stretched by applying tension until it was fractured. The loads and the extension were measured by means of a load cell and extensometer, respectively. During the application of tension, the elongation of the gauge section was recorded against the applied force.
2.6 Hardness testing
Brinell hardness test was conducted at the Federal Institute of Industrial Research Oshodi (FIIRO), Lagos State, Nigeria. Brinell hardness was measured on polished surface of samples using a load of 980 N for the entire test. Three readings were taken for each sample and the average hardness was determined using the following formula:
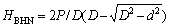 (1)
(1)
where HBHN is the Brinell hardness, P is the load applied, D is the diameter of indenter, and d is the diameter of indentation.
3 Results
3.1 Effect of concentration of wetting agent and cleaning time on tensile strain
Variations in strain at break (a measure of ductility) of AA6011/SiCp with cleaning time and concentration of NaCl are shown in Fig. 1. Samples containing SiCp washed in 40 g/L NaCl solution display the best response to tensile deformation between 80 and 120 min of cleaning time followed by 20 g/L NaCl. At a cleaning time of 80 min, the maximum ductility for Al/SiCp reached as it strained to 9.3% at break for 40 g/L NaCl treatment solution. MAZAHERY and SHABANI [26] reported that the addition of SiCp deteriorated the ductility of A356 alloy.
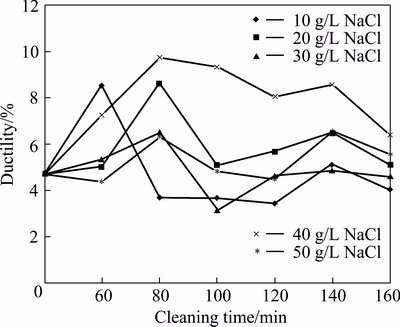
Fig. 1 Ductility of AA6011/SiCp with cleaning time and concentration of NaCl
There is a continuous decrease in strain of AA6011/SiCp with the use of 5 g/L NH4Cl between 0 and 160 min of cleaning time, as shown in Fig. 2. However, there are fluctuations in deformation response for composites having SiCp cleaned with 15 and 30 g/L of the same reagent of NH4Cl, where a maximum strain of 11.2% is attained with NH4Cl concentration of 30 g/L at 60 min.
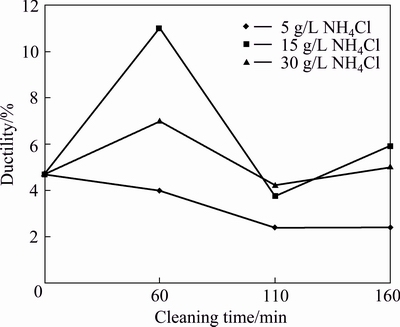
Fig. 2 Ductility of AA6011/SiCp with cleaning time and concentration of NH4Cl
Figure 3 shows that the ductility of AA6011/SiCp increases with cleaning time of the reinforcement when using 1 g/L PdCl2 cleaning reagent, and the maximum strain at break is 11.6% at 120 min. Comparing these results with the control sample (cleaning time of 0), cleaning SiCp with PdCl2 for 160 min (least strain at break of 3%) lowers the ductility of AA6011/SiCp by 60%. This observation could be attributed to poor surface property of the SiCp induced during prolonged cleaning. Surface irregularities may have developed, resulting in poor bonding between matrix and filler.
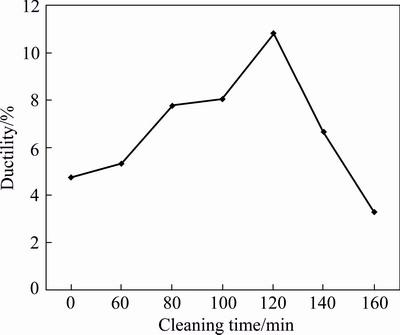
Fig. 3 Ductility of AA6011/SiCp with cleaning time using 1 g/L PdCl2
Cleaning SiCp with SnCl2 for 60 min promotes significant increase in ductility of AA6011/SiCp samples produced in the order of concentrations: 50 g/L<30 g/L< 20 g/L<10 g/L<40 g/L (see Fig. 4). The lowest ductility of AA6011/SiCp reached at 20 and 30 g/L SnCl2 for 120 and 80 min, respectively, with similar strain at break of 2.1%.
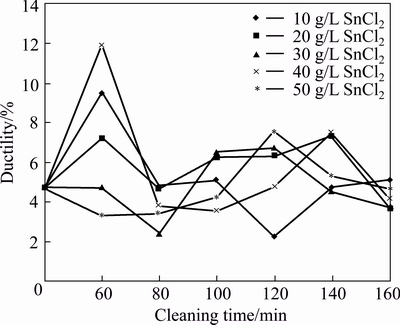
Fig. 4 Ductility of AA6011/SiCp with cleaning time and concentration of SnCl2
3.2 Effect of cleaning concentration and time on composite hardness
The hardness of AA6011/SiCp influenced by cleaning time and concentrations of NaCl on SiCp filler is shown in Fig. 5. When compared with the control sample, it is observed that cleaning the carbide with NaCl leads to reduction in hardness. However, exceptions occur at 40 g/L NaCl (for 120 min) and 50 g/L NaCl (for 100 min) where hardnesses increased by 6.3% and 7.9%, respectively. The observed decline in hardness may be linked with weak interface between filler and matrix that permit easy dislocation motion, reduction in Al3C4 and absence of particle clustering. It is assumed that the reinforcement cleaned with lower concentration of the reagent leads to interfacial asperities hindering effective filler/matrix interfacial bonding, thus becoming a weak point to permit easy movement of dislocations.
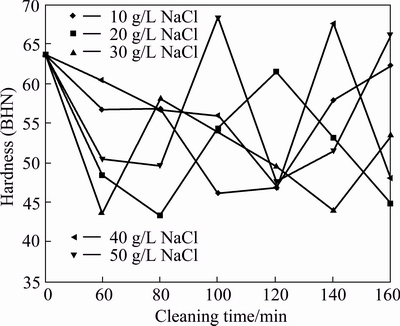
Fig. 5 Hardness of AA6011/SiCp with cleaning time and concentration of NaCl
A progressive reduction in hardness is observed with 15 g/L NH4Cl as opposed to sinusoidal fluctuations of hardness observed for 30 g/L NH4Cl (Fig. 6). Improved hardness is shown by composites with SiCp cleaned with 30 g/L between 110 and 160 min cleaning time, with maximum hardness of BHN 61 at 110 min. Thus, these cleaning reagents promote the decrease of the hardness while the control AA6011/SiCp has superior hardness of BHN 63.76.
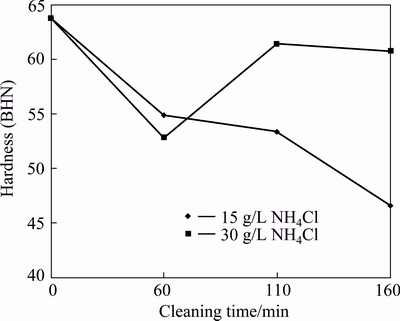
Fig. 6 Hardness of AA6011/SiCp with cleaning time and concentration of NH4Cl
Figure 7 shows the effect of SiCp cleaning time with 1 g/L PdCl2 on hardness of AA6011/SiCp. The least hardness value of BHN 40, which is 36% lower than that of the control specimen, is attained at 100 min cleaning time, while BHN 65 is obtained in composite with SiCp cleaned for 160 min. This maximum hardness is higher than that of the control specimen (BHN 63).
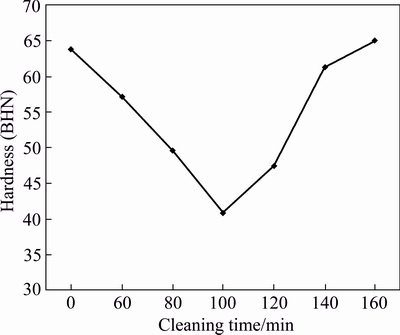
Fig. 7 Hardness of AA6011/SiCp with cleaning time at 1 g/L PdCl2
There is fluctuation in hardness of AA6011/SiCp, which is caused by variations in SnCl2 concentrations and cleaning time (see Fig. 8). At 80 min, the peak hardness is BHN 185 at 30 g/L SnCl2. Considering the cleaning time, reagents with 20 and 40 g/L SnCl2 reduced the hardness of the composite less than that of the control composite.
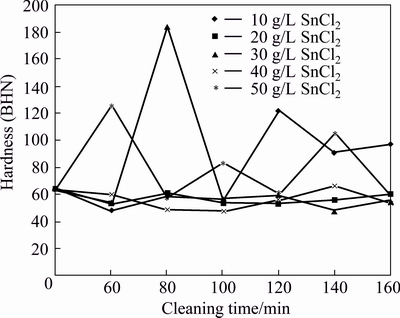
Fig. 8 Hardness of AA6011/SiCp with cleaning time and concentration of SnCl2
4 Discussion
All composites with SiCp cleaned using NaCl solution show the same behaviour with initial rise in tensile strain to a maximum before further decline. The microstructures (Fig. 9) of the composite with SiCp cleaned using 10 g/L NaCl reveal the reasons for this behaviour. The morphology of the composites at 60 min cleaning time (see Fig. 9(a)) shows SiCp fairly dispersed in the microstructure of α(Al) matrix. The same features are observed by YILMAZ [27] while investigating the effect of SiCp on 7xxx series aluminium alloys. The structure shows that few SiCp particles are properly bonded to the matrix, and this explains the initial increase in tensile strain shown in Fig. 1. The presence of Fe-rich intermetallic precipitates is noticed on the surface of the morphologies. These Fe-rich intermetallics were loosely bonded to the Al-matrix, resulting in a decline in the hardness of composites (see Fig. 5). The primary cause of reduction in hardness is the increase in micro-porosity and possible reaction at the interface [28]. Figure 9(b) shows the microstructure of composites with SiCp cleaned for 100 min. The structure shows few SiCp particles on the surface (see Fig. 9(b)) with few Fe-rich intermetallic crystals. Few SiCp particles are found to be clustered at a point. This is considered to be the cause of decrease in the tensile strain of composite (see Fig. 1). Figure 9(c) shows the microstructure of composites with SiCp cleaned for 160 min. The microstructure shows considerable amount of SiCp sparsely dispersed in the Al matrix with few clusters. It is noticed that the clusters have weak interface with the matrix, resulting in matrix cracking. Figure 1 shows that the strain of this composite is lower than that of other composites. MALLICK et al [28] noted earlier that interfacial reaction is helpful for load transfer from the matrix to SiCp. However, excessive interfacial reaction is detrimental to the mechanical properties of the composites as thicker interfacial zone facilitates de-bonding. The poor interfacial interaction is attributed to the effect of the cleaning process and cleaning time.
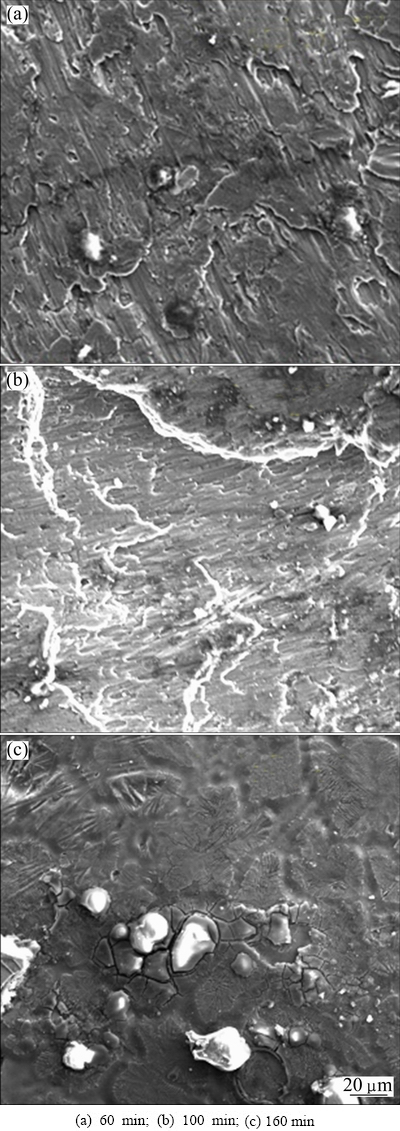
Fig. 9 Microstructures of AA6011/SiCp cleaned with 10 g/L NaCl for different cleaning time
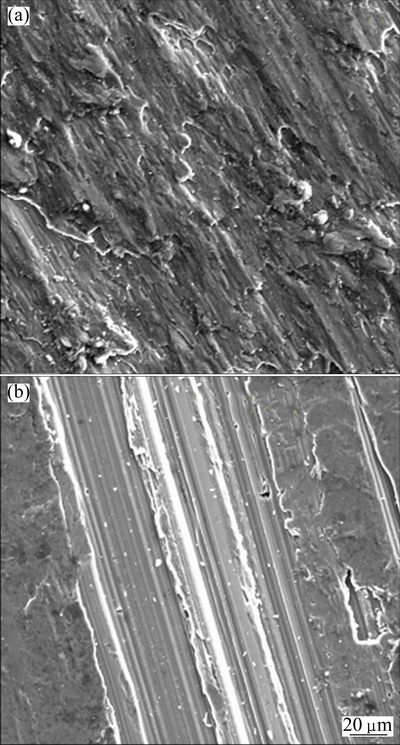
Fig. 10 Microstructures of AA6011/SiCp cleaned with 15 g/L NH4Cl for 60 min (a) and 5 g/L NH4Cl for 100 min (b)
Composites with particles cleaned with NH4Cl solution show superior tensile strain at a concentration of 15 g/L for 60 min. This increase in tensile strain is attributed to the fair distribution of SiCp and AlFeSi intermetallic precipitates, which are strongly bonded to the matrix, as shown in Fig. 10(a). On the other hand, the marked low ductility of the composites with particles cleaned with 5 g/L NH4Cl solution for 100 min is attributed to the reduction in the free SiCp on the matrix surface and the presence of large cracks (see Fig. 10(b)). The microstructure shows the presence of few SiCp scattered over the surface with very fine intermetallic precipitates of AlFeSi. The presence of large crack is also evident in the microstructure, which can contribute to the reduction in ductility.
PdCl2-cleaned composites show a marked effect of cleaning time on the tensile strain of the composites. There is an increase in tensile strain with cleaning time to a maximum at 120 min before decline with the increases in time. This trend is explained by the difference in the microstructure of the composites shown in Fig. 11. The initial increase in tensile strain at 60 min cleaning time is attributed to the presence of few SiCp with large Fe-containing intermetallic precipitates embedded in the microstructure (see Fig. 11(a)). Fe-bearing intermetallic precipitates such as β-Al5FeSi and α-Al12 (Fe,Mn)3Si are typically found in 6xxx alloys and these have significant influence on the formability. The round cubic α-AlFeSi phase results in superior deformability and ductility. A marked increase in ductility to a maximum value was noted when the cleaning time was increased to 120 min. The microstructure shows that increasing cleaning time to 120 min gives increase precipitation of intermetallic precipitates of AlFeSi and Mg2Si (see Fig. 11(b)). Large volume fraction of long needle-shaped primary Fe-rich intermetallic is also formed and dispersed over the microstructure. The formation of these intermetallics during die-casting of Al-Mn-Si alloys was early noted by JI et al [29]. The dendritic structure tends to give way to lamellar structure with Mg2Si arranged along the grain boundaries. Few SiCp particles are observed on the surface of the composites. It is believed that the combination of these features promotes the improvement in tensile strain (see Fig. 3). It is evident that there is good interfacial bonding between SiCp and α(Al) matrix. These features are lost with further increase in reinforcement cleaning time to 160 min (see Fig. 11(c)). Large dendritic structure is shown with AlFeSi precipitates occupying the dendritic arms, which explains the sharp decrease in ductility of the composites.
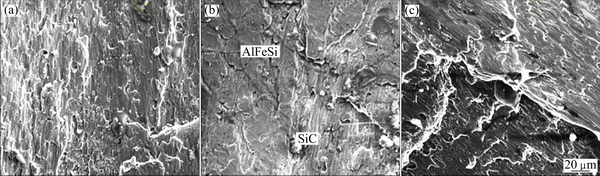
Fig. 11 Microstructures of AA6011/SiCp cleaned with 1 g/L PdCl2 for 60 min (a), 120 min (b) and 160 min (c)
Composites with SiC particles cleaned with SnCl2 show superior properties at a concentration of 40 g/L. The improvement of properties in this set of composites is attributed to the influence of the cleaning agent and time on the final microstructure of the composites. Figure 12(a) shows the microstructure at 60 min cleaning time of the reinforcement. The microstructure shows high volume fraction of SiCp distributed over the surface with some crystals of Fe-containing intermetallic. The cast structure is dendritic but shows very short dendritic arms. SiCp is strongly bonded to the matrix and located between the dendrite arms. This could account for the increase in tensile strain of the composite (see Fig. 4). The AlFeSi precipitates are globular and embedded in the matrix. This is an indication that fast cooling occurs at the onset of solidification. PANAHI [30] reported that at high cooling rates, globular precipitates are formed, which are distributed over the microstructure of AlFeSi alloys. It is believed that the globular precipitates of AlFeSi enhance the ductility. The increase in cleaning time to 100 min (see Fig. 12(b)) shows the presence of few SiCp on the microstructure with needle-like Fe-containing intermetallic. The matrix consists of leaf-like layers laid on each other. The layers are large (over 200 μm) in size but bonded together at the edges. Some clusters of AlFeSi precipitates are also evident. These features confer marked decrease on the ductility of the composites (see Fig. 4). Further increase in cleaning time presents morphology with considerable amount of SiCp on the surface together with few tiny intermetallic precipitates (see Fig. 12(c)). SiCp is poorly bonded to the matrix, which may be the reason for the low tensile strain (see Fig. 4). The differences in structural features of the composites are probably due to the interference of SiCp with the matrix and the solidification process.

Fig. 12 Microstructures of AA6011/SiCp cleaned with 40 g/L SnCl2 for 60 min (a), 100 min (b) and 160 min (c)
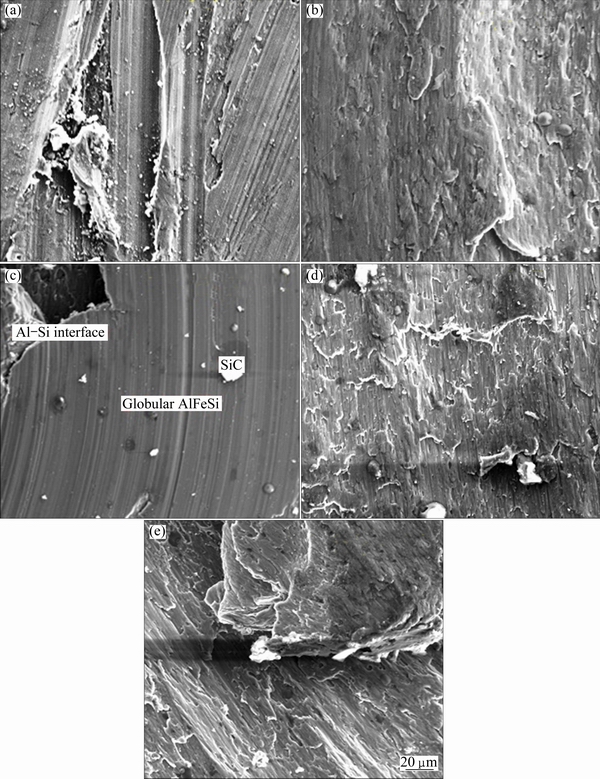
Fig. 13 Microstructures of AA6011/SiCp cleaned with 10 g/L SnCl2 (a), 20 g/L SnCl2 (b), 30 g/L SnCl2 (c), 40 g/L SnCl2 (d) and 50 g/L SnCl2 (e) for 60 min
The comparison of microstructures of composites at different SnCl2 concentrations (see Fig. 13) shows the reason for the superior tensile strain of 40 g/L SnCl2 over that of other concentrations. The microstructure of 40 g/L SnCl2 (Fig. 13(d)) shows large volume fraction of SiCp over that of Figs. 13(a), (b), (c) and (e). Moreover, the cast structure is dendritic with very short dendritic arms. The microstructure (Fig. 13(d)) also reveals that the SiCp is strongly bonded to the matrix and located between the dendrite arms which accounts for the superior tensile strain of the composite over that of 10, 20, 30 and 50 g/L SnCl2. Although the microstructure of 10 g/L SnCl2 (Fig. 13(a)) shows an ordered structure, it is evident that the presence of large volume fraction of Fe-containing intermetallic particles found on the surface hinders ductility. These precipitates are less than 10 μm in average size and are not so visible. Composites with 20 g/L SnCl2 (see Fig. 13(b)) contain very few SiCp dispersed on the microstructure with a few needle-like Fe-containing intermetallic precipitates which account for the moderate tensile strain displayed by the composite. On the other hand, composite with 30 g/L SnCl2 shows very weak interfacial bonding between SiCp and α(Al) matrix (see Fig. 13(c)). The low ductility, compared with that of 40 g/L SnCl2, is attributed to the poor bonding. The microstructure of composite with filler cleaned with 50 g/L SnCl2 contains small volume fraction of SiCp in a dendritic cast structure. Few intermetallic precipitates are also found in the microstructure. The structure also suggests very large sizes of dendrites which account for a low ductility compared with that at 40 g/L SnCl2.
5 Conclusions
Based on the experimental evidences from this research, SnCl2, PdCl2, NaCl and NH4Cl are found useful wetting agents for the production of AA6011/SiCp composites with improved ductility and hardness characteristics. These wetting agents are effective in improving interfacial bonding at the matrix/ reinforcement interface and inhibiting the formation of Al4C3 which is a detrimental precipitate to the mechanical properties of AA6011/SiCp composite. The order of effectiveness of the wetting agents is SnCl2>PdCl2>NaCl>NH4Cl. NaCl shows superior hardness and ductility responses with 40 g/L concentration at 100 min cleaning time, PdCl2 with 1 g/L concentration at 120 min, SnCl2 with 40 g/L concentration at 60 min and NH4Cl with 15 g/L concentration for 60 min. The best combination of hardness (BHN 57.88) and ductility (11.91%) is shown at 40 g/L SnCl2 and cleaning time of 60 min.
Acknowledgement
The authors acknowledge with grateful thanks for the University of Lagos, Nigeria, for providing the platform for the Tetfund Research Grant (CRC/ TETFUND/No.2011/2013) used for this research. We are also grateful for the assistance provided by Mr. W. A. Ayoola, Mr. Olajide Kehinde and the staff of Metallurgical and Materials Engineering Laboratory and Foundry shop of University of Lagos.
References
[1] CHEN J K, HUANG I S. Thermal properties of aluminum–graphite composites by powder metallurgy [J]. Composites: Part B, 2013, 44: 698-703.
[2] RAJMOHAN T, PALANIKUMAR K, RANGANATHAN S. Evaluation of mechanical and wear properties of hybrid aluminium matrix composites [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(8): 2509-2517.
[3] ADEOSUN S O, AKPAN E I, ABIODUN D. Mould temperature and mechanical properties of cast aluminum-silicon carbide composite [J]. International Journal of Materials and Chemistry, 2013, 3(4): 75-83.
[4] PRABHU B, SURYANARAYANA C, ANA L, VAIDYANATHAN R. Synthesis and characterization of high volume fraction Al–Al2O3 nanocomposite powders by high-energy milling [J]. Materials Science and Engineering A, 2006, 425: 192-200.
[5] KHODAI M M, RAJABI M, ASKARI N, MIRHADI B, JANIPOUR B, OVEISI H. Nano-composites produced by microwave sintering [C]//Proceedings of International Aluminium Conference, 2012. Arak, Iran, 2012.
[6] TIKHOV S F, PAKHOMOV N A, NEMYKINA E I, SALANOV A N, SADYKOV V A, ROMANENKOV V E, PIATSIUSHYK Y Y. Porous ceramic matrix Al2O3/Al composites as supports and precursors for catalysts and permeable materials [C]//Metal, Ceramic and Polymeric Composites for Various Uses. New York: Intech, 2011: 195-210.
[7] BAGHCHESARA M A, ABDIZADEH H, BAHARVANDI H R. Fractography of stir cast Al-ZrO2 composites [J]. Iranian Journal of Science and Technology, 2009, 33(B5): 453-462.
[8] BAREKAR N, TZAMTZIS S, DHINDAW B K, PATEL J, BABU N H, FAN Z. Processing of aluminum-graphite particulate metal matrix composites by advanced shear technology [J]. Journal of Materials Engineering and Performance, 2009, 18(9): 1230-1240.
[9] NAGLIERI V, PALMERO P, MONTANARO L, CHEVALIER J. Elaboration of alumina-zirconia composites: Role of the zirconia content on the microstructure and mechanical properties [J]. Materials, 2013, 6(5): 2090-2102.
[10] MURPHY A M, HOWARD S J, CLYNE T W. Characterisation of severity of particle clustering and its effect on fracture of particulate metal matrix composites [J]. Material Science and Technology, 1998, 14: 959-968.
[11] SEGURADO J, BAHARVANDI C X, LORCA J L. A numerical investigation of the effect of particle clustering on the mechanical properties of composites [J]. Acta Materialia, 2003, 51: 2355-2369.
[12] ZAKERIA M, RUDI A V. Effect of shaping methods on the mechanical properties of Al-SiC Composite [J]. Materials Research, 2013, 16(5): 1169-1174.
[13] DENG X, CHAWLA N. Modeling the effect of particle clustering on the mechanical behavior of SiC particle reinforced Al matrix composites [J]. J Mater Sci, 2006, 41: 5731-5734.
[14] TZAMTZIS S, BAREKARA N S, BABUA N H, PATELA J, DHINDAW B K, FANA Z. Processing of advanced Al/SiC particulate metal matrix composites under intensive shearing—A novel rheo-process [J]. Composites: Part A, 2009, 40: 144-151.
[15] LING C P, BUSH M B, PERERA D S. The effect of fabrication on techniques on the properties of Al-SiC composites [J]. Journal of Materials Processing Technology, 1995, 48: 325-331.
[16] HONG S J, KAO P W. Mechanical properties of Al-SiC composites made by resistance sintering of mechanically alloyed powders [J]. Materials Science and Engineering A, 1991, 148(1): 89-195.
[17] ROSTAMZADEH T, SHAHVERDI H R. Microstructure study on Al-5%SiC nanocomposite powders [J]. Iranian J Mat Sci Engr, 2011, 8(1): 32-39.
[18] ROSTAMZADEH T, SHAHVERDI H, SHANAGHY A. EIS study of bulk Al-SiC nanocomposite prepared by mechanical alloying and hot press method [J]. Adv Mat Res, 2010, 83: 1297-1305.
[19] ADEOSUN S O, AKPAN E I, BALOGUN S A, ABDULMUNIM A S. Tin (II) chloride a suitable wetting agent for AA1200-SiC composites [J]. International Journal of Chemical, Nuclear, Metallurgical and Materials Engineering, 2014, 8(4): 307-301.
[20] PRAKASH O, SANG H, EMBURY J D. Structure and properties of A1-SiC foam [J]. Materials Science and Engineering A, 1995, 199: 195-203.
[21] JISHNU J B, MITRA R. Effect of hot rolling temperature and thermal cycling on creep and damage behavior of powder metallurgy processed Al-SiC particulate composite [J]. Materials Science and Engineering A, 2012, 557: 92-105.
[22] SOBCZAK N, KSIAZEK M, RADZIWILL W, MORGIEL J, BALIGA W, STOBIERSKI L. Effect of titanium on wettability and interfaces in the Al/ SiC system [C]//Proceedings of the International Conference on High Temperature Capillarity. Cracow, Poland, 1997.
[23] LAURENT V, CHATAIN D, EUSTATHOPOULOS N. Wettability of SiC by aluminium and Al-Si alloys [J]. J Mater Sci, 1987, 22(1): 224-250.
[24] ALONSO A, GARCIA-CORDOVILL C, LOUIS E, NARCISO J. Evaluation of the wettability of Al-Pb and Al-Sn alloys with SiC and Al2O3 particulates by means of pressure infiltration [J]. J Mater Sci, 1994, 29(18): 4729-4735.
[25] CAROTENUTO G, GALLO A, NICOLAIS L. Superficial modification of SiC powder by chemical oxidation [J]. Appl Comp Mat, 1994, 1(2): 155-166.
[26] MAZAHERY A, SHABANI M O. Characterization of cast A356 alloy reinforced with nano SiC composites [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(1): 275-280.
[27] YILMAZ H S. Characterization of silicon carbide particulate reinforced squeeze cast aluminum 7075 matrix composite [D]. Ankara: Middle East Technical University, Turkey, 2004.
[28] MALLICK B, MAITY P C, SINHA V K. Composite materials— Manufacture, processing, evaluation, application and technologies [C]//National Metallurgical Laboratory 1998: Production of Aluminium-Silicon Carbide Cast Particle Composites without Magnesium. Jamshedpur, India, 1998.
[29] JI S, WENCHAO Y, FENG G, DOUGLAS W, ZHONGYUN F. Effect of iron on the microstructure and mechanical property of Al-Mg-Si-Mn and Al-Mg-Si die cast alloys [J]. Materials Science and Engineering A, 2013, 564: 130-139.
[30] PANAHI D. Precipitation of intermetallic phases from rapidly solidifying aluminum alloys [D]. Hamilton: McMaster University, Canada, 2009.
氯化物净化处理AA6011/SiCp复合材料的延展性和硬度
S. O. ADEOSUN1, E. I. AKPAN2, O. P. GBENEBOR1, S. A. BALOGUN3
1. Department of Metallurgical and Materials Engineering, University of Lagos, Lagos 100213, Nigeria;
2. Department of Materials and Production Engineering, Ambrose Alli University, Ekpoma 310101, Nigeria;
3. Department of Mechanical and Biomedical Engineering, Bells University of Technology, Ota 110001, Nigeria
摘 要:以NaCl、SnCl2、NH4Cl和PdCl2为润湿剂对SiCp进行净化处理。以净化处理后的SiCp为增强体,采用搅拌铸造法制备AA6011/Sip复合材料。采用标准方法对复合材料的延展性和硬度进行研究,并分别采用扫描电镜和X射线衍射仪研究复合材料的显微组织和相组成。结果表明,对于所有的润湿净化剂,复合材料的延展性随净化时间的延长增大到一定值,然后随着净化时间的继续延长,其延展性反而减小。在净化剂SnCl2浓度为40 g/L和净化时间为60 min的条件下,复合材料获得硬度为BHN 57.88和延展性为11.91%的最佳综合性能。复合材料的X射线衍射谱中仅出现少量Al4C3,表明净化过程抑制了有害析出物的形成。
关键词:铝合金复合材料;碳化硅;润湿剂;延展性;硬度;增强体
(Edited by Wei-ping CHEN)
Corresponding author: E. I. AKPAN; E-mail: emma_eia@yahoo.com
DOI: 10.1016/S1003-6326(16)64124-9
Abstract: The ductility and hardness of AA6011/SiCp composites using NaCl, SnCl2, NH4Cl and PdCl2 as wetting reagents were investigated. SiCp was cleaned with the wetting reagents, and used as reinforcement in AA6011 alloy using the stir casting method. Ductility and hardness responses of the composites were measured using standard methods. Microstructural features were examined using scanning electron microscopy and the phases were determined with the help of an X-ray diffractometer. The results show that for all wetting agents, the increase in cleaning time leads to initial increase in ductility to a certain value, but a decrease afterwards with further increase in cleaning time. The best combination of hardness (BHN 57.88) and ductility (11.91%) was shown under conditions of 40 g/L SnCl2 and cleaning time of 60 min. A minor formation of Al4C3 was noted in diffraction patterns, indicating that the formation of deleterious precipitate was hindered by the cleaning process.


