J. Cent. South Univ. (2016) 23: 1052-1057
DOI: 10.1007/s11771-016-0354-y

Tungsten removal from molybdate solutions using chelating ion-exchange resin: Equilibrium adsorption isotherm and kinetics
ZHU Xian-zheng(朱先正), HUO Guang-sheng(霍广生), NI Jie(倪捷), SONG Qiong(宋琼)
School of Metallurgy and Environment, Central South University, Changsha 410083, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
The equilibrium adsorption isotherm and kinetic of the sorption process for W and Mo on macro chelating resin D403 were investigated on single Na2MoO4 and Na2WO4 solutions. The sorption isotherm results show that the adsorption process of W obeys the Freundlich model very well whereas the exchange process with Mo approximately follows the Henry model. The kinetic experiments show that the intraparticle diffusion process was the rate-determining step for W sorption on the resin, and the corresponding activation energy is calculated to be 21.976 kJ/mol.
Key words:
tungsten; molybdate; ion-exchange resin; isotherms; kinetics;
1 Introduction
The separation between W and Mo has been a national difficulty due to their extremely similar physicochemical properties, resulting from the lanthanide contraction [1]. Additionally, according to the industrial requirement, WO3 impurity in final ammonium molybdate product should be restricted to below 150×10-6 (mass fraction) [2], making W removal from molybdate solutions particularly important.
Currently, the reported approaches to separating W from molybdate solutions mainly lie in changing the properties of W or Mo compounds and exploiting these property differences [3-7]. By taking advantage of the difference in tendency to polymerize at pH around 6.5-7.5 between Mo and W, basic adsorbents particularly those containing tertiary amino groups such as extractants (N263, N1923) [8-9] or macroporous anion exchangers (D301, D309) [10-11] were investigated and presented good W removal efficiency. Specifically, at pH 6.5-7.5, W in aqueous solutions would be preferentially polymerized into polymeric ions  while Mo still mainly exists as monomeric ions
while Mo still mainly exists as monomeric ions  [12]. As a result of their higher electrovalence, W polymeric ions
[12]. As a result of their higher electrovalence, W polymeric ions  would be preferentially adsorbed using anion adsorbents. However, the difficulty to control pH values within such a narrow range of 6.5-7.5, to a certain extent, limits its further industrial use. Besides, some other researches also proposed to add precipitation agents, such as the divalent ions (Mn2+, Fe2+) [7, 13] and tin (IV) oxide hydrate [14], that would preferentially form precipitations with W under certain conditions. The separation was effective but a large number of precipitation agents were required and the impurity ions accompanied with the added reagents were inevitable, increasing the impurity-removal burden.
would be preferentially adsorbed using anion adsorbents. However, the difficulty to control pH values within such a narrow range of 6.5-7.5, to a certain extent, limits its further industrial use. Besides, some other researches also proposed to add precipitation agents, such as the divalent ions (Mn2+, Fe2+) [7, 13] and tin (IV) oxide hydrate [14], that would preferentially form precipitations with W under certain conditions. The separation was effective but a large number of precipitation agents were required and the impurity ions accompanied with the added reagents were inevitable, increasing the impurity-removal burden.
Inspired by the different affinities towards O between W and Mo, we discovered a novel resin (D403) containing meglumine group which exhibited marvelous separation effects between Mo and W at pH above 8.0, at which W and Mo would mainly exist as monomeric ions,  and
and  This finding indicates that this resin could be directly utilized to treat the industrial molybdate solutions without adding any reagents or even adjusting pH values, which was further confirmed through the column tests on the mock industrial ammonium molybdate solutions with W removal rates up to 97.56% [15].
This finding indicates that this resin could be directly utilized to treat the industrial molybdate solutions without adding any reagents or even adjusting pH values, which was further confirmed through the column tests on the mock industrial ammonium molybdate solutions with W removal rates up to 97.56% [15].
In order to further reveal the separation mechanism of D403 resin and provide some instructions for the practical production, the researches involving single-component isothermal adsorption and adsorption kinetic experiments were investigated and simulated in this work.
2 Experimental
2.1 Materials
The macro polyhydroxy chelating resin D403 (shown in Table 1) resembling Amberlite IRC-743, which contains the meglumine group and has been widely used to extract boric acid was collected from Jiangsu Suqing industrial Co., Ltd, Jiangsu, China. Prior to testing, the resin was successively pretreated with 5% HCl solution, 5% NaOH and then washed to be near-neutral. All the chemicals used in this work were of analytical grade. All the concentrations of W and Mo were detected using inductively coupled plasma-atomic emission spectrometer (ICP-AES, Thermo Electron corporation, US).
Table 1 Physical properties of D403 resin in this study

2.2 Adsorption isotherm experiments
In the adsorption isotherm experiments, 10 mL D403 wet resin was mixed with 50 mL solution including differently concentrated single Na2WO4 and Na2MoO4 solution in water-bath shaker for 4 h at room temperature. According to the previous study [15], the pH values of all the solutions were adjusted to 9.25 with hydrochloride acid in advance.
Similar to other chemical processes, the ion exchange equilibrium can be characterized by corresponding equilibrium isotherms. In this case, the adsorption equilibrium of W and Mo will be simulated with Henry model [16], Langmuir model [17] and Freundlich model [18]. The mathematical expressions of the three models are described as follows:
Henry model:  (1)
(1)
Freundlich model:  (2)
(2)
Langmuir model:  (3)
(3)
where Qe is the equilibrium adsorption capacity of wet resin (mg/cm3); Ce is concentration of the equilibrium solutions after the exchange process (mg/dm3); Qm is the maximum adsorption capacity of the resin (mg/cm3); KH, KF, b, n are the corresponding constant parameters of these models.
The adsorbed capacity of the resin at equilibrium Qe (mg/cm3) can be calculated as follows:
 (4)
(4)
where C0 and Ce are the initial and equilibrium metal concentrations, respectively; V, VR are the volumes of tested solution and the resin, respectively.
2.3 Adsorption kinetic experiments
In order to ensure the ISV (infinite solution volume) kinetic condition, namely to maintain the nearly constant concentration of metals in solutions, only few wet resin (2 mL, particle radius of 0.6-1.0 mm) was added into abundant solutions (500 mL, single Na2MoO4 solutions: 0.3 mol/dm3 Mo; single Na2WO4 solutions: 0.1 mol/dm3 WO3). The experiments were carried out at various temperatures, and the solutions were sampled at certain intervals and then detected.
The aim of the adsorption kinetic experiments is to figure out the possible rate-determining steps of the exchanging process, and three main steps are considered, namely, diffusion of ions through the liquid film, chemical reaction process and diffusion of ions inside the materials. Dominance of one of these mechanisms can be predicted using the following approximate criterion [19].
Film diffusion control:
 (5)
(5)
Particle diffusion control:
 (6)
(6)
Chemical reduction control:
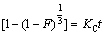 (7)
(7)
where F is the exchange rate of the resin, which is determined by
 (8)
(8)
where KF, KP and KC are the apparent rate constants of the corresponding models.
3 Results and discussion
3.1 equilibrium adsorption isotherms
The experimental data of the equilibrium adsorption for W and Mo on the D403 resin are presented in Fig. 1, showing that the equilibrium adsorption quantity of both W and Mo increases with the increasing metal concentration in solutions, and that equilibrium curve for W is obviously higher than that for Mo, which indicates a stronger affinity of D403 towards W than towards Mo. Additionally, the S-shaped equilibrium curve for Mo is across the diagonal line, which means that there are two different function groups affecting the adsorption of Mo [20-21]. Referring to our previous contrast tests for the removal of W between 403 resin (tertiary amino groups and hydroxy groups) and D301 resin (tertiary amino groups) [15], we can make a reasonable prediction that the dominant working groups of the resin are hydroxy groups when C/Cm<0.35, while when C/Cm>0.35, tertiary amino groups of the resin begin to play a more important role.

Fig. 1 Single-component equilibrium adsorption of W and Mo with D403 at pH 9.25 and 25 °C (where Cm is the maximum equilibrium concentration of adsorption)
To better understand the adsorption of W and Mo, Henry model, Freundlich model and Langmuir model were utilized to fit the isotherm data (shown in Figs. 2-4). The fitting results show that the adsorption for W fits the Freundlich model well and the equilibrium equation is: lnQ=0.385lnC+2.914, R2=0.962. This means that the corresponding isotherm constant 1/n is equal to 0.385 (below 0.5), indicating the strong affinity of the resin towards W. However, the sorption for Mo follows Henry model well, getting sorption equation: Q=1.198C+2.209, R2=0.984. As we know, if the slope of the Henry line (KH) is equal to 1.0, this means the resin has little affinity towards the target ions. In the case of the KH constant for Mo here, 1.198 is only little above 1.0, which indirectly proves the weak affinity towards Mo. In conclusion, the isotherm fitting results further illustrate the stronger affinity of the D403 resin towards W than towards Mo, which is consistent with our previous findings. Also these equations will be favorable for plants to predict the equilibrium capacity of the resin for different solutions.
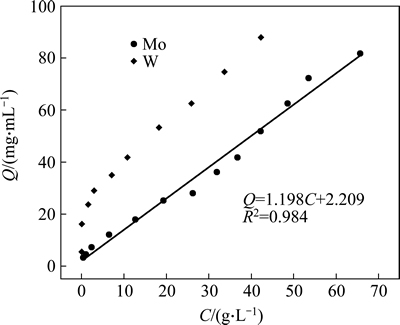
Fig. 2 Henry isotherm plot for adsorption of W and Mo on D403 resin
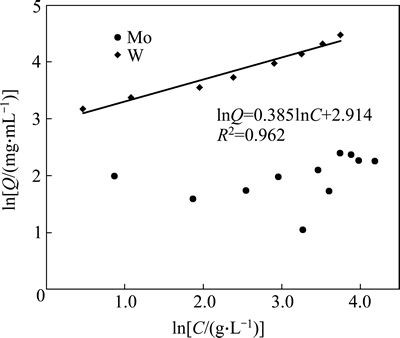
Fig. 3 Freundlich isotherm plot for adsorption of W and Mo on D403 resin

Fig. 4 Langmuir isotherm plot for adsorption of W and Mo on D403 resin
3.2 sorption kinetic
The experiments in terms of kinetics for W and Mo were carried out on single Na2WO4 (0.1 mol/dm3 WO3) and single Na2MoO4 (0.3 mol/dm3 Mo) solutions at different temperatures (25, 30, 45, 60 °C). The maximum capacity of the resin for W and Mo is tested in advance with saturation capacity calculated to be 33.23 mg/cm3 (Mo) and 189.25 mg/cm3 (WO3).
It is evident that the adsorption rates for both W and Mo rise with the increasing reaction temperature, which indicates that the exchange process for both W and Mo is endothermic, and that raising temperature is beneficial for the sorption of target ions. In addition, the results in Fig. 5 show that the sorption for W reaches saturation state at around 350 min, which is faster than that for Mo.
The experimental data were simulated with the widely used kinetic models and the results in Figs. 6-8illustrate that sorption for W is controlled by the particle diffusion process with linearly dependent coefficients above 0.99. However, for Mo there are still no proper models which could fit the experimental data well.

Fig. 5 Adsorption kinetics of Mo (a) and W (b) with D403 at different temperatures
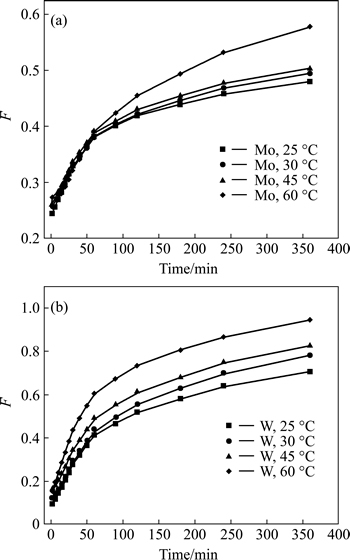
Fig. 6 Film diffusion control model plots for adsorption of Mo (a) and W (b) with D403 resin at different temperatures

Fig. 7 Particle diffusion control model plots for adsorption of Mo (a) and W (b) with D403 resin at different temperatures
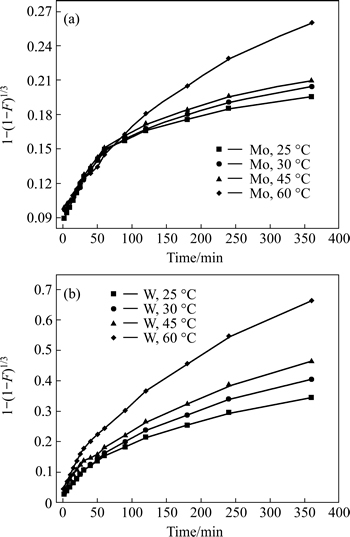
Fig. 8 Chemical reaction control model plots for adsorption of Mo (a) and W (b) with D403 resin at different temperatures
3.3 Adsorption activation energy
Above analysis shows that the adsorption process of W with D403 was controlled by diffusion in the particle. The slop of the line in Fig. 7 presents the apparent rate constant of reaction (K) and the values are 0.00080, 0.00108, 0.00128, 0.00220 respectively. The gradually increasing slop value illustrates that the ion exchange is the endothermic reaction further more. To investigate the activation energy of adsorption, the plot of lnK versus 1000/T is shown in Fig. 9. Then the activation energy (E) can be determined by the Arrhenius equation:
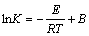 (9)
(9)
Calculated statistics show that the activation energy of adsorption equilibrium of W is only 21.976 kJ/mol, which is below <42 kJ/mol, indicating that the adsorption process for W is controlled by a diffusion mechanism [22-24].
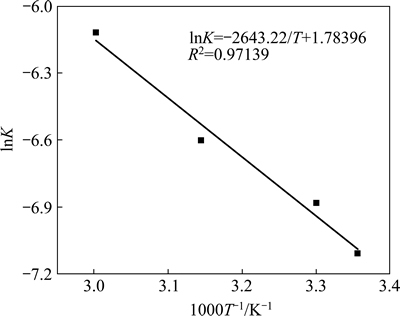
Fig. 9 Arrhenius plot of adsorption equilibrium of W with D403 resin.
4 Conclusions
1) In order to further reveal the separation mechanism of D403 resin and provide some instructions for the practical production, adsorption isotherm and kinetics for W and Mo on D403 resin were investigated.
2) The isotherm simulation results show that the adsorption for W fits the Freundlich model well and the equilibrium equation is: lnQ=0.385lnC+2.914, R2=0.962. The isotherm constant 1/n (0.385) is below 0.5 which indicates a strong affinity of the resin towards W. However, the sorption for Mo follows Henry model well, getting sorption equation: Q=1.198C+2.209, R2=0.984. This means that the distribution coefficient for Mo is only 1.198, indicating a weak affinity of the resin towards Mo.
3) The kinetic experiments show that the exchange process for both W and Mo is endothermic, and that raising temperature is beneficial for the sorption of target ions. The adsorption process for W is controlled by the diffusion in the particles, and the corresponding activation energy is calculated to be 21.976 kJ/mol.
References
[1] WIBERG E, WIBERG N, HOLLEMAN A F. Inorganic chemistry [M]. San Diego: Academic Press, 2001: 1385-1386.
[2] GB/T 3460. Ammonium molybdate [S]. 2007. (in Chinese)
[3] ZHAO Zhong-wei, CAO Cai-fang, CHEN Xing-yu. Separation of macro amounts of tungsten and molybdenum by precipitation with ferrous salt [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(12): 2758-2763.
[4] TALLA R G, GAIKWAD S U, PAWAR S D. Solvent extraction and separation of Mo(VI) and W(VI) from hydrochloric acid solutions using cyanex-923 as extractant [J]. Indian Journal of Chemical Technology, 2010, 17(6): 436-440.
[5] GUAN Wen-juan, ZHANG Gui-qing, GAO Cong-jie. Solvent extraction separation of molybdenum and tungsten from ammonium solution by H2O2-complexation [J]. Hydrometallurgy, 2012, 127(1): 84-90.
[6] LI Hong-gui, HUO Guang-sheng, ZHAO Zhong-wei. Developing new reagent for selectively precipitation of molybdenum from tungstate solution [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(1): 184-187.
[7] ZHAO Zhong-wei, CAO Cai-fang, CHEN Xing-yu, HUO Guang-sheng. Separation of macro amounts of tungsten and molybdenum by selective precipitation [J]. Hydrometallurgy, 2011, 108(3): 229-232.
[8] KE Zhao-hua, ZHANG Gui-qing, GUAN Wen-juan, SHANG Guang-hao. Research on tungsten extraction from alkali sodium tungstate solution with quaternary ammonium salt [J]. Rare Metals and Cemented Carbides, 2012, 40(1): 1-4. (in Chinese)
[9] NING Peng-ge, CAO Hong-bin, ZHANG Yi. Selective extraction and deep removal of tungsten from sodium molybdate solution by primary amine N1923 [J]. Separation and Purification Technology, 2009, 70(1): 27-33.
[10] LU Xiao-ying, HUO Guang-sheng, LIAO Chun-hua. Separation of macro amounts of tungsten and molybdenum by ion exchange with D309 resin [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 3008-3013.
[11] ZHAO Zhong-wei, ZHANG Jia-liang, CHEN Xing-yu, LIU Xu-heng, LI Jiang-tao, ZHANG Wei-guang. Separation of tungsten andmolybdenum using macroporous resin: equilibrium adsorption for single and binary systems [J]. Hydrometallurgy, 2013, 140: 120-127.
[12] SHEKHTER L N, LITZ J E, WEI Xiong. Separation of tungsten from ammonium molybdate solutions: US, 20130243673A1 [P]. 2013-09-19.
[13] ZHANG Jia-liang, ZHAO Zhong-wei, CHEN Xing-yu. Thermodynamic analysis for separation of tungsten and molybdenum in W-Mo-H2O system. [J] The Chinese Journal of Nonferrous Metals, 2013, 23(5): 1463-1470. (in Chinese)
[14] CHERESNOWSKY, MICHAEL J. Method for separating tungsten from molybdenum: US, 4999169 [P]. 1991-03-12.
[15] HUO Guang-sheng, PENG Chao, SONG Qiong, LU Xiao-ying. Tungsten removal from molybdate solutions using ion exchange [J]. Hydrometallurgy, 2014, 147: 217-222.
[16] Rengaraj S, JOO C K, KIM Y, YI J. Kinetic of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H [J]. Journal of Hazardous Materials, 2003, 102(2): 257-275.
[17] LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum [J]. Journal of American Chemical Society, 1918, 40(9): 1361-1403.
[18] FREUNDLICH H M F. Over the adsorption in solution [J]. J Phys Chem, 1906, 57: 385-470.
[19] LIAO Chun-hua. The new technology for separation of tungsten and molybdenum by ion exchange [D]. Changsha: central south university, 2012. (in Chinese)
[20] LAZARIN A M, BORGO C A, GUSHIKEM Y, KHOLIN Y V. Aluminum phosphate dispersed on a cellulose acetate fiber surface: Preparation, characterization and application for Li+, Na+ and K+ separation [J]. Analytica Chimica Acta, 2003, 477(2): 305-313.
[21] ANDERI Z A. Zagorodni. Ion exchange materials: Properties and Applications [M]. New York: Elsevier, 2006.
[22] ZHANG Jia-liang, LIU Xu-heng, CHEN Xing-yu, LI Jiang-tao, ZHAO Zhong-wei. Separation of tungsten and molybdenum using macroporous resin: Competitive adsorption kinetics in binary system [J]. Hydrometallurgy, 2014, 144: 77-85.
[23] AL-GHOUTI M, KHRAISHEH M A M, AHMAD M N M, ALLEN S. Thermodynamic behaviour and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: a kinetic study [J]. Journal of Colloid and Interface Science, 2005, 287(1): 6-13.
[24] DING Ping, HUANG Ke-long, LI Gui-yin, LIU Yan-fei, ZENG Wen-wen. Kinetics of adsorption of Zn(II) ion on chitosan derivatives [J]. International Journal of Biological Macromolecules, 2006, 39(4): 222-227.
(Edited by YANG Hua)
Foundation item: Project(2014CB643405) supported by National Research Development Program of China
Received date: 2015-11-04; Accepted date: 2016-03-08
Corresponding author: HUO Guang-sheng, Professor, PhD; Tel: +86-18507312882; E-mail: gshuo@csu.edu.cn
Abstract: The equilibrium adsorption isotherm and kinetic of the sorption process for W and Mo on macro chelating resin D403 were investigated on single Na2MoO4 and Na2WO4 solutions. The sorption isotherm results show that the adsorption process of W obeys the Freundlich model very well whereas the exchange process with Mo approximately follows the Henry model. The kinetic experiments show that the intraparticle diffusion process was the rate-determining step for W sorption on the resin, and the corresponding activation energy is calculated to be 21.976 kJ/mol.


