
Properties of electrodeposited amorphous Fe-Ni-W alloy deposits
HE Feng-jiao(何凤姣)1, WANG Miao(王 淼)1, LU Xin(陆 欣)2
1. College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China;
2. Yingcai Technology Co. Ltd, Changsha 410006, China
Received 9 March 2006; accepted 22 July 2006
Abstract:
A new technique of electroplating amorphous Fe-Ni-W alloy deposits was proposed. The structure and morphology of Fe-Ni-W alloy deposit were detected by XRD and SEM. The friction and wear behavior of Fe-Ni-W alloy deposit were studied and compared with that of chromium deposit. The corrosion properties against 5% sodium chloride, 5% sulfuric acid and 5% sodium hydroxide were also discussed. The experimental results indicate that Fe-Ni-W alloy deposits have superior properties against wear than hard chromium deposits under dry sliding condition. Under oil sliding condition, except their better wear resistance, the deposits can protect their counterparts against wear. The deposits plated on brass and AISI 1045 steel show good behavior against corrosion of 5% sodium chloride, 5% sulfuric acid and 5% sodium hydroxide. The bath of electroplating amorphous Fe-Ni-W alloy deposits is environmentally friendly and would find widely use in industry.
Key words:
electrodeposition; amorphous Fe-Ni-W alloy deposits; wear resistance; corrosion resistance;
1 Introduction
Chromium electrodeposition is a widely applied technique in industries for the production of decorative and functional coatings. That is because it possesses attractive properties such as decorative appearance, high hardness, and excellent wear and corrosion resistance. However, it also possesses some shortcomings such as low cathode current efficiency(CCE) (only 12%-15% in industry production), low throwing and covering power of plating bath, high current density (20 A/dm3), high tank voltage and high energy consumption[1]. Furthermore, chromium plating solutions are corrosive, toxic and carcinogenic because of the hexavalent chromium and the sulfate acid[2]. The plating process produces large amount of chrome contaminated toxic waste and causes health problem such as skin and lung irritation. It requires special disposal methods[3]. As a result, the plating industries have been forced to consider other materials which the bath is highly effective and environmentally friendly.
Compared with chromium plating technique, electroplating tungsten alloy technique has its own advantages, such as high cathode current efficiency (above 70%), good throwing and covering power, low current density (3-8 A/dm3) and environmentally friendly electrolyte. So the co-deposition of tungsten with one or more of iron group metals provides a number of electrodeposits that might be considered possible replacement for chromium. A lot of paper reported the electrodeposition of binary tungsten alloys (Co/Ni/Fe-W) [4-16], but for the ternary tungsten alloys very few reported[12,13,17,18]. In the present research, many people discussed the effects of electroplating parameters and bath composition on deposits, but nobody discussed the wear and corrosion properties of ternary tungsten alloys, especially the wear mechanisms under different sliding conditions. In this paper, a new technique of electroplating Fe-Ni-W alloy from aqueous solution was proposed. The properties of deposits against wear and corrosion were studied in detail. A comparative study of hard chromium deposits under the same testing condition was also made.
2 Experimental
2.1 Electroplating of Fe-Ni-W alloy deposits
The bath and the process parameters of electro- plating Fe-Ni-W alloy deposits are shown in Table 1.
Table 1 Bath and parameters for electroplating Fe-Ni-W alloy deposits
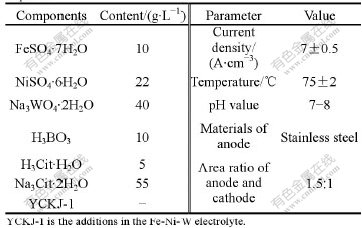
The amorphous Fe-Ni-W alloy deposits were plated on the surface of AISI 1045 steels and copper alloys. The deposits were obtained through the following procedures: burnishing—polishing—degreasing—wash- ing with hot water—washing with cold water—weak corrosion with acid—washing—electroplating. The thickness of the deposit was determined by micrometer.
2.2 Heat treatment
The heat treatment for the deposits was carried out in air at temperature of 540 ℃ in a muffle furnace. The alloy deposit samples were put in the furnace, maintained for 40 min, and then quenched in air to gain higher hardness and better wear resistance.
2.3 Determination of composition and characteriza- tion of Fe-Ni-W alloy deposit
After electroplating, the composition of Fe-Ni-W alloy deposit was determined. The content of tungsten in layers was determined with a Leng Guang 722 visual spectrophotometer, the contents of iron and nickel in layers were determined with WFX-1C atomic absorption spectrophotometer. The morphologies of Fe-Ni-W alloy deposits were observed using a KYKY-2800 scanning electron microscope(SEM). The microstructure of the layer after heat treatment was tested by a Japan Rigaku D/max-rA X-ray diffractometer(XRD) employing Cu target, Kα radiation.
2.4 Hardness measurement
The hardness was measured with a HVS-1000 digital microhardness tester. The load was 1.96 N and the loading time was 10 s. Six points on the surface of Fe-Ni-W deposit were chosen randomly to determine the hardness and the average value was taken.
2.5 Wear tests
The wear tests were carried out with a SST-ST disk friction and wear testing machine (West Germany). The tests under dry sliding and N150 lubrication environment were run. The other experimental conditions were as follows: the counterpart was AISI 52100 standard steel ball with the diameter of d8 mm, the sample was Fe-Ni-W alloy of about 40 μm thickness plated on AISI 1045 steel with the size of d50 mm×8 mm. The sample deposits and their counterparts were burnished by CC 2000 abrasive paper and degreased with absolute alcohol before wear test. The sliding velocity was 0.21 m/s, the environmental temperature was 15 ℃ and humidity was 50%-65%. For dry friction, only 50 N loading was chosen and for N150 oil friction, three different loadings (50 N, 100 N, 150 N) were chosen. The chromium deposits were detected under the same condition for comparing. The morphologies of the deposits and their counterparts after wear tests were obtained in-situ during the testing experiment. The friction coefficient was calculated through the recording data of moment and the wear resistance property was calculated by linear wear capacity, which was the total thickness loss of the sample deposit and its counterpart.
2.6 Corrosion tests
Fe-Ni-W alloy deposits with the thickness of 15 μm were plated on AISI 1045 steel and brass substrate. The corrosion resistance of the deposits was studied by neutral salt spray experiment (5% NaCl, (35±1)℃, pH=6.5-7.2, continuous spray), dipping in sulfuric acid (5% H2SO4 at room temperature) and sodium hydroxide (5% NaOH at room temperature).
3 Results and discussion
3.1 Morphology, composition and structure of electroplating tungsten alloy
The morphology of deposit is shown in Fig.1. The deposit is even and compact. Some micro cracks exist on the surface of the deposit, which is caused by its high internal stress. The components of alloy deposit shown in Fig.2 are in accordance with that determined by spectrophotometer. The contents of iron, nickel, tungsten

Fig.1 SEM image of Fe-Ni-W deposit
in the deposit are 40%, 25%, 35% (mole fraction), respectively. The X-ray diffraction shows that the structure of the layer after heat treatment at the temperature of 540 ℃ is still in amorphous phase state (Fig.3). At this temperature, the alloy deposit cannot change to crystal. The structure of as-deposited layer is amorphous even the content of tungsten is low to 25%. This is different from that of Fe-W, Co-W or Ni-W deposit reported by in Refs.[14-16]. It is said that the microstructure of deposit is dependent on the content of tungsten in deposit. When the content of tungsten is lower, the structure of deposits is crystalline. But when tungsten content reaches about 70% in Fe-W[14], 35% in Co-W[15], 42% in Ni-W alloy[16], the deposits change to the amorphous state. So it can be concluded that for ternary alloys, the tungsten content in the deposits to form amorphous is less than that in binary alloys.
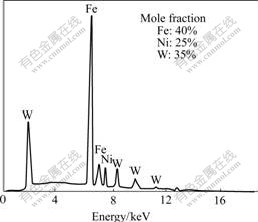
Fig.2 EDX spectrum for Fe-Ni-W deposit
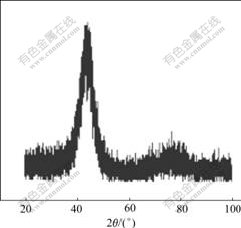
Fig.3 XRD pattern of Fe-Ni-W deposit after heat treatment at 540 ℃
3.2 Properties of tungsten alloy deposits
3.2.1 Hardness
The Vickers hardness of as-deposited layer is about HV600. After heat treatment at the temperature of 540 ℃, the Vickers hardness can reach HV1300. This is higher than that of chromium deposit. The high hardness of the Fe-Ni-W deposit may be attributed to its structure and composition. After heat treatment at the temperature of 540 ℃ the deposit forms new amorphous state without any hydrogen in the deposit, which improves the hardness of the deposit and makes the surface of the layer even and compact. So it is of some wonderful properties compared with crystal structure, especially high hardness, good wear and corrosion resistance. The content of tungsten in the deposit also affects its hardness. The higher the content of tungsten is, the higher the hardness.
3.2.2 Adhesion
The deposits have good adhesion to the carbon steel and copper alloys. Either chiseled by file or annealed, the deposits could not peel off the substrates.
3.2.3 Wear resistance
The experimental results under dry sliding condition are shown in Fig.4. Under the 50 N loading condition, the friction coefficient of chromium is about 0.24 at first, it increases sharply after 300 s; but for the Fe-Ni-W deposit, the friction coefficient is about 0.22 and increases after 3 100 s. Obviously, under 50 N loading and dry sliding condition, the wear resistance of Fe-Ni-W deposit is better than that of chromium deposit. The morphologies of deposits after wear test confirm this result (see Fig.5). Only slight nicks and narrow blurry wear belt are observed on the Fe-Ni-W deposit surface. The surfaces of the deposits and its counterparts are not absolutely even, there are many hard micro particles appeared on the deposits and its counterparts. Under the dry sliding condition, the particles can pile into the deposit and produce nicks on the surface of the deposit. The wear mode is particle wear. The Fe-Ni-W alloy deposit is still amorphous after heat treatment at the temperature of 540 ℃, the new amorphous phase improves the hardness and makes the surface of the deposit more slippery than that of chromium deposit. So
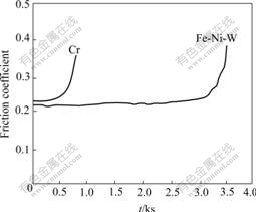
Fig.4 Friction coefficients of chromium and Fe-Ni-W deposit under dry sliding conditions
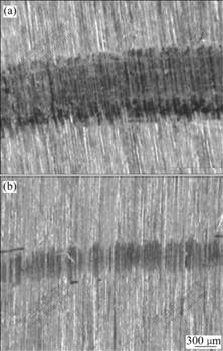
Fig.5 Wear morphologies of Cr(a) and Fe-Ni-W(b) deposits under dry sliding conditions
the number of the particles produced during sliding is less than that of chromium deposit, Fe-Ni-W alloy deposit is worn more lightly than chromium deposit under dry sliding condition.
Under oil sliding condition, three different loadings were used to detect the properties of deposits against wear. The friction coefficients and wear capacities are shown in Figs.6 and 7. The friction coefficients of Fe-Ni-W samples are 0.11-0.14, and those of chromium deposit are 0.12-0.14. So under N150 lubrication condition, the friction coefficients of Fe-Ni-W deposits are equivalent to those of chromium deposits (see Fig.6). The linear wear capacities of Fe-Ni-W deposits and their counterparts are 1-4 μm, and those of chromium deposits and their counterparts are 7-10 μm. So the linear wear capacities of Fe-Ni-W deposits are father lower than those of the chromium (see Fig.7). The Fe-Ni-W deposits show much better wear resistance than chromium deposits under oil sliding condition.
The morphologies of the deposits and their counterparts after test are shown in Figs.8 and 9. The results shows that under three loadings, there is some difference in wear property for Fe-Ni-W and chromium deposits, but significant difference for their counterparts. Chromium deposits and their counterparts wear out more seriously than Fe-Ni-W deposits and their counterparts. After wear resistance test under oil sliding condition, the surfaces of chromium deposits show dense and deep furrow nicks, and the nicks become clear with the increase of load. But there are only slight marks on the
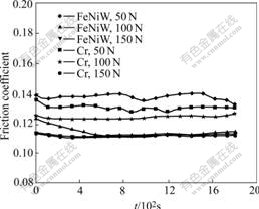
Fig.6 Friction coefficients of two deposits under oil sliding conditions
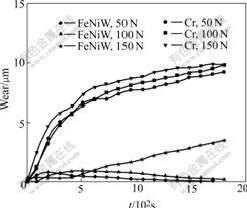
Fig.7 Linear wear capacities of two deposits under oil sliding conditions
surfaces of the Fe-Ni-W deposits. The surfaces of chromium deposits are much rougher than those of Fe-Ni-W deposits. The amorphous phase of Fe-Ni-W alloy deposit after heat treatment makes the deposit surface even, uniform and exquisite. In the course of friction, the exquisite surface can protect the counterparts of the Fe-Ni-W deposits. That is why there are only slight wear marks on the surface of the Fe-Ni-W counterparts. The wear mode of Fe-Ni-W deposit and its counterpart under this condition is particle wear. Otherwise, the surface of chromium plating is quite rough and there are many micro bulges on the surface. When loading and sliding, the micro bulges adhere to the counterparts and form adherence points. During sliding process, the adherence points could cut the counterparts and produce chippings peeled off from the counterparts. So we could see peeling holes on the surfaces of the chromium counterparts. The wear mode of the chromium deposit and its counterpart under this condition is adhesive wear. The number of the peeling holes is increased with the increase of load and the counterparts
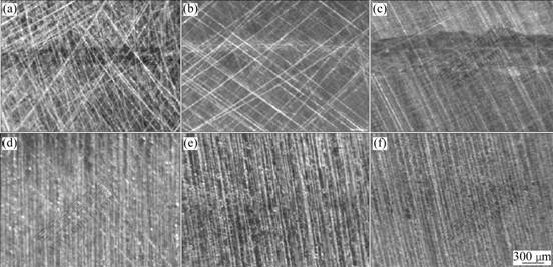
Fig.8 Wear morphologies of two deposits under oil sliding conditions: (a) FeNiW, 50 N; (b) FeNiW, 100 N; (c) FeNiW, 150 N; (d) Cr, 50 N; (e) Cr, 100 N; (f) Cr, 150 N
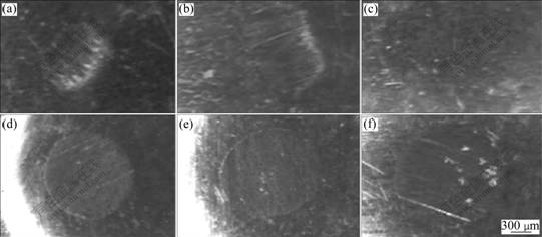
Fig.9 Wear morphologies of their counterparts under oil sliding conditions: (a) FeNiW, 50 N; (b) FeNiW, 100 N; (c) FeNiW, 150 N; (d) Cr, 50 N; (e) Cr, 100 N; (f) Cr, 150 N
of chromium deposits wear heavily under three different loads. Another reason for the fine wear resistance of Fe-Ni-W alloy deposits under oil sliding condition may be that lots of micro cracks on the surfaces of Fe-Ni-W deposits (see Fig.1) can store the N150 lubricant, which can protect the counterparts and decrease the wear capacities of Fe-Ni-W deposits. Therefore, under this condition, Fe-Ni-W deposits are not only wear resistant but also protect their counterparts against wear.
3.2.4 Corrosion resistance
The corrosion behavior of Fe-Ni-W alloy deposits is shown in Table 2. It can be seen that the kinds of substrates and the thickness of the deposit can affect the corrosion resistance against sodium chloride and sulfuric acid, but have no effect on corrosion resistance against sodium hydroxide. The corrosion resistance of Fe-Ni-W layers deposited on brass substrates is good against sodium hydroxide, sulfuric acid and sodium chloride. Those deposited on the AISI 1045 steel substrates are good against sodium hydroxide, but a little poor against sodium chloride and sulfuric acid. The reason is that much thinner deposits (about 15 μm) can not protect substrates efficiently. The deposits with thickness above 25 μm can protect AISI 1045 steel substrates against sodium chloride and sulfuric acid. Because of the new amorphous structure, the deposit is uniform. There are no dislocation, disfigurement and interface in the amorphous phase. So the amorphous structure of Fe-Ni-W deposits after heat treatment shows good corrosion behavior against sodium hydroxide, sulfuric acid and sodium chloride solution.
4 Conclusions
1) The hardness of amorphous Fe-Ni-W alloy deposit can reach to HV1 300 after heat treatment at the temperature of 540 ℃. The deposits have good adhesion to different substrates, such as AISI 1045 steels and copper alloys.
Table 2 Corrosion resistance of Fe-Ni-W alloy deposits on different substrates

2) Under dry sliding condition, the chromium deposit is worn more heavily than Fe-Ni-W alloy deposit. The amorphous phase of Fe-Ni-W deposit after heat treatment improves the hardness and its wear resistance. The wear mode of the both deposits is particle wear. Under oil sliding condition, the friction coefficients of Fe-Ni-W deposits are similar to those of chromium deposits. The linear wear capacities of Fe-Ni-W alloy deposits are farther lower than those of chromium deposits. The counterparts for chromium are worn more seriously than those of Fe-Ni-W deposits. The exquisite surface of Fe-Ni-W deposit can protect its counterpart against wear. Therefore, Fe-Ni-W alloy deposits are not only wear resistant, but also protect their counterparts against wear. Under this condition, the wear mode of Fe-Ni-W deposit is particle wear and that of chromium deposit is adhesive wear.
3) The amorphous structure of Fe-Ni-W deposits after heat treatment shows good behavior against corrosion of 5% sodium chloride, 5% sulfuric acid and 5% sodium hydroxide. The deposit plated on AISI 1045 steel with thickness above 25 μm can protect the substrate against sodium chloride and sulfuric acid.
4) Fe-Ni-W deposit not only possesses better properties than those of chromium deposit, but also is environmentally friendly and low cost. It could be widely used in industry for replacement of chromium deposits.
Acknowledgement
The authors would thank Professor LI Jian (Institute of Materials Protection of Wuhan) for the detection of wear resistance.
References
[1] ZENG Hua-liang, WU Zhong-da, CHEN Jun-wu. Handbook of Electroplating Techniques [M]. Beijing: China Machine Press, 1997. (in Chinese)
[2] GUAN Shan, ZHANG Qi, HU Ru-nan. Recent development of chromium electrodeposition [J]. Materials Protection, 2000, 33(3): 1-3. (in Chinese)
[3] SCHELL J D, RECHSTEINER M. Replacement of chromium electroplating using advanced material technologies on gas turbine engine components [J]. Plating and Surface Finishing, 2000, 87(7): 17-23.
[4] ABDEL-HAMID Z. Electrodeposition of cobalt-tungsten alloys from acidic bath containing cationic surfactants [J]. Materials Letters, 2003, 57(16/17): 2558-2564.
[5] DONTEN M, GROMULSKI T, STOJEK Z. The interface between metallic substrates and layers of electrodeposited Co-W amorphous alloys [J]. Journal of Alloys and Compounds, 1998, 279(2): 272-278.
[6] SCHLOBMACHER P, YAMASAKI T. Structural analysis of electroplated amorphous-nanocrystalline Ni-W [J]. Mikrochim Acta, 2000, 132(2/4): 309-313.
[7] YOUNES-METZLER O, ZHU L, GILEADI E. The anomalous codeposition of tungsten in the presence of nickel [J]. Electrochimica Acta, 2003, 48(18): 2551-2562.
[8] SHAHIN G E. Alloys are promising as chromium or cadmium substrates [J]. Plating and Surface Finishing, 1998, 85(8): 8-14.
[9] SOMEKAWA H, NIEH T G, HIGASHI K. Instrumented indentation properties of electrodeposited Ni-W alloys with different microstructures [J]. Scripta Materialia, 2004, 50(11): 1361-1365.
[10] REN Rong, WU Yu-cheng, SHU Xia, SHI Cheng-wu, LI Yun, ZHENG Yu-chun. Microstructure and performance of electro- deposited nanocrystalline Ni-W alloys [J]. Trans Nonferrous Met Soc China, 2005, 15(s3): 198-202.
[11] YAMASAKI T, TOMOHIRA R, OGINO Y, SCHLOSSMACHER P, EHRLICH K. Formation of ductile amorphous and nanocrystalline Ni-W alloys by electrodeposition [J]. Plating and Surface Finishing, 2000, 87(5): 148-152.
[12] DONTEN M, CESIULIS H, STOJEK Z. Electrodeposition and properties of Ni-W, Fe-W and Fe-Ni-W amorphous alloys: A comparative study [J]. Electrochimica Acta, 2000, 45(20): 3389-3396.
[13] CAPEL H, SHIPWAY P H, HARRIS S J. Sliding wear behavior of electrodeposited cobalt-tungsten and cobalt-tungsten-iron alloys [J]. Wear, 2003, 255(20): 917-923.
[14] NASU T, SAKURAI M, KAMIOYAMA T, USUKI T, UERNURA O, TOKUMITSU K, YAMASAKI T. Structural comparison of M-W (M=Fe, Ni) alloys produced by electrodeposition and mechanical alloying [J]. Mater Sci Eng A, 2004, 375-377(complete): 163-170.
[15] SVENSSON M, WAHLSTR?M U, HOLMBOM G. Composi- tionally modulated cobalt-tungsten alloys deposited from a single ammoniacal electrolyte [J]. Surface and Coatings Technology, 1998, 105(3): 218-223.
[16] WU Y Y, CHANG D Y, KIM D S, KWON S C. Effects of 2-butyne-1, 4-diol on structures and morphologies of electroplating Ni-W alloy [J]. Surface and Coatings Technology, 2003, 162(2/3): 269-275.
[17] HE Feng-jiao, LEI Jing-tian, LU Xin, HUANG Yu-ning. Friction and wear behavior of electrodeposited amorphous Fe-Co-W alloy deposits [J]. Trans Nonferrous Met Soc China, 2004, 14(5): 901-906.
[18] GAO Cheng-hui, ZHAO Yuan. Wear mechanism of electrodeposited amorphous Ni-Fe-P alloys [J]. Trans Nonferrous Met Soc China, 2004, 14(2): 255-259.
(Edited by YUAN Sai-qian)
Foundation item: Project(04GK1007) supported by the Science and Technology Office of Hunan Province, China
Corresponding author: HE Feng-jiao; Tel/Fax: +86-731-8914786; E-mail: Fengjiao863@hotmail.com


